Abstract
Psoriatic disease (PsD) is a multisystem inflammatory disorder with a high prevalence of cardiovascular (CV) risk factors contributing to accelerated atherosclerosis and its sequelae. Imaging studies, notably with ultrasound, computed tomography, and positron emission tomography (PET) scanning have confirmed significant atherosclerotic change with plaque formation and vessel stenosis. Atherosclerosis is likely driven by a combination of traditional risk factors which occur more frequently in PsD and by systemic inflammation with associated pro-inflammatory cytokine production. While the mechanisms driving atherosclerosis in PsD are incompletely understood, it is now best practice to try to minimize the impact of CV risk factors by regular assessment, prevention, and treatment and also by ensuring that inflammatory musculoskeletal and cutaneous disease is adequately suppressed. Future studies need to focus on improving our understanding of the mechanisms driving atherosclerosis and, as a consequence, developing more rationale approaches to prevention and treatment.
Similar content being viewed by others
Patients with psoriatic disease (PsD) have an increased prevalence of traditional cardiovascular risk factors and are at greater risk of cardiovascular morbidities and subsequent major cardiovascular events compared to the normal population. |
Atherosclerosis is likely driven by a combination of traditional risk factors and by systemic inflammation with associated pro-inflammatory cytokine production. |
Vascular imaging studies with ultrasound, CT, and PET scanning have confirmed higher burden of atherosclerotic change with plaque formation and vessel stenosis in PsD as compared to the general population that is independent of traditional risk factors. |
Rheumatologists should take responsibility for cardiovascular risk assessment in patients with PsD |
Introduction
Psoriatic arthritis (PsA) complicates cutaneous psoriasis in about 30% of cases. The clinical features of the musculoskeletal component of PsA can be quite diverse with patients presenting with one or more domains of involvement including peripheral arthritis, axial inflammation, dactylitis, or enthesitis. Extra-musculoskeletal inflammatory disease can also occur including uveitis and inflammatory bowel disease. The concept of psoriatic disease (PsD) has been proposed in an effort to convince dermatologists and rheumatologists to view the disease in all the ways that a patient can be effected rather than in the more traditional, speciality-specific manner. Persistent inflammatory disease in a patient with PsD contributes to a deterioration in patient’s health and well-being including an effect on function, work, income and social participation. In this review, we will focus on cardiovascular comorbidities in PsD, initially examining the role of inflammation in atherosclerotic plaque formation. We will look in detail at the evidence for increased cardiovascular disease (CVD) in PsD and at the risk factors for its development. Finally, we will discuss issues related to risk factor prevention and suggest areas for future research.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Cardiovascular Comorbidities in PsD
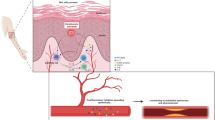
Pathogenesis of Atherosclerosis
Atherosclerosis is a chronic inflammatory CVD, which results in one of the most common causes of death in the older population. Atherosclerosis is characterized by lipid deposition in the arterial wall with smooth muscle cell and fibrous matrix proliferation, resulting gradually over time in the development of an atherosclerotic plaque. The development of atherosclerotic plaques is the pathological basis of CVD whereby unstable plaques rupture resulting in platelet aggregation and thrombosis, subsequent stenosis, or blockage of blood vessels, leading to acute CVD events such as myocardial infarction (MI) or cerebrovascular accident. Traditional atherosclerotic risk factors (e.g., dyslipidemia and hypertension) and novel risk factors (e.g., the systemic inflammation associated with arthritis) contribute to the development of CVD in rheumatoid arthritis (RA) and similar factors are thought to pertain to PsD [1].
Endothelial dysfunction, with oxidative stress and macrophage accumulation, and subsequent pro-inflammatory cytokine production, such as tumor necrosis factor alpha (TNFα), interleukin (IL)-1β and IL-6, are a few of the mechanisms implicated in plaque development. Supporting this concept, there is accumulating evidence that targeted biologic drugs, such as TNF inhibitors and anti-IL1β agents, can slow the atherogenic process. Thus, early and effective control of inflammatory disease, in addition to aggressive management of classical cardiovascular risk factors, may result in both short- and long-term benefit to the patient with prevention of the consequences of atherosclerosis [2].
Cytokines such as TNFα, IL-1β, and IL-6 are critically involved in the inflammatory process related to PsD and both circulating cytokines and cytokines produced locally within the vessel wall may help drive the atherosclerotic process. It is of interest that pro-inflammatory pathways, in particular inflammasome signaling, was seen in brachial vein-derived endothelial cells from patients with skin psoriasis [3]. Furthermore, the changes seen in the endothelium in terms of pro-inflammatory cytokine production bore a striking resemblance to that seen in the more traditionally involved skin and synovial tissue.
IL-17 and associated cytokines such as IL-23 also appear critical to the inflammatory process in PsD, a statement in particular supported by the success of targeted therapies blocking these cytokines, while major players in the initiation and progression of atherosclerosis [4], IL-17-producing lymphocytes, also known as T helper (h)17 cells, have been shown to have both pathogenic and non-pathogenic roles [5]. To date, follow-up of IL-17 and IL-23 inhibitor randomized controlled trial data does not suggest a significant concern relating to cardiovascular events but more long-term analysis may well shed more light on this important area of controversy.
Burden of Cardiovascular Disease in PsD
Patients with PsD are at increased risk of cardiovascular morbidities and are at greater risk for subsequent major cardiovascular events compared with the general population [6]. A population-based cohort study including over 130,000 patients with psoriasis and 550,000 controls from the General Practice Research Database in the United Kingdom revealed that psoriasis was an independent risk factor for MI and the adjusted relative risk (RR) for MI was greatest in young patients with severe psoriasis [7]. Using the same source, Metha and colleagues found that patients with severe psoriasis have an increased risk of cardiovascular mortality that is independent of traditional cardiovascular risk factors [8]. In line with these results, a systematic review and meta-analysis of 14 different psoriasis cohorts and across different disease severities reported an increased risk of CVD only in individuals with severe psoriasis, the RR was 1.37 [95% confidence interval (CI) 1.17–1.60] for CVD mortality, 3.04 (95% CI 0.65–14.35) for MI, and 1.59 (95% CI 1.34–1.89) for stroke [9].
Although there are less data regarding cardiovascular risk factors and cardiovascular comorbidity in patients with PsA compared with that in psoriasis, most studies point towards an increased cardiovascular risk in PsA, broadly on par with the risk level in RA [10]. It has been suggested that the increased cardiovascular risk in PsA is associated with a combination of traditional cardiovascular risk factors and disease activity [11]. A recent systematic review and meta-analysis of observational studies indicated that people with PsA have a 43% increased risk of CVD and a 55% increased risk of developing incident cardiovascular events compared with the general population and that the magnitude of the elevated risk is similar to that observed in patients with severe psoriasis [12]. In a Danish nationwide cohort study on psoriasis-related cardiovascular morbidity and mortality, psoriasis was a significant cardiovascular risk factor independent of age, gender, comorbidity, concomitant medication, and socio-economic status. The risk of cardiovascular events and mortality was age-dependent and higher amongst younger patients with severe psoriasis and was similar in patients with severe psoriasis to those with psoriatic arthritis [13]. Furthermore, in a large population-based longitudinal study quantifying the risk of major adverse cardiovascular events (MACE) including MI, cardiovascular death and stroke among patients with PsA, RA, and psoriasis after adjustment for traditional cardiovascular risk factors, PsA patients who had not received disease-modifying antirheumatic drugs (DMARDs) had a higher risk of MACE compared to controls, similar to those with severe psoriasis and RA patients not prescribed a DMARD [14]. Despite increased cardiovascular risk being well recognized in patients with PsD, it is not accurately captured by traditional risk assessment [15, 16] and there remains an unmet need to develop appropriate risk factor assessment and screening for CVD in this population.
Evidence for Atherosclerotic Vascular Change in PsD
Carotid Ultrasound Studies
Vascular ultrasound studies have shown a higher prevalence of carotid artery plaques and vascular inflammation in patients with PsD compared with unaffected controls [17]. Carotid duplex ultrasound is a non-invasive imaging technique which can identify the presence of carotid plaques representing an unequivocal manifestation of atherosclerosis and serving as a surrogate for CV disease [18]. In most studies, the extent of atherosclerosis is quantified by measuring total plaque area (TPA), carotid intima-media thickness (cIMT), and plaque category. A handful of studies have shown that cIMT is higher in patients with psoriasis and PsA as compared to healthy controls [19, 20]. It has been suggested that PsA patients suffer from more severe subclinical atherosclerosis compared to patients with psoriasis alone and the difference is independent of traditional CV risk factors and correlates with markers of increased inflammation [21]. Another study including patients from the Toronto PsA cohort found that higher burden of inflammation is associated with more severe atherosclerosis. Surprisingly, the authors did not find an association between disease duration and the extent of atherosclerosis, which was likely due to the high prevalence of traditional CV risk factors that may be present before the onset of PsA [17]. While cIMT is a reliable marker of atherosclerosis, data on its correlation with risk of future CV events is limited. A recent study including 559 patients with PsD aimed at assessing whether subclinical carotid atherosclerosis, as evaluated by cIMT and total TPA, could predict incident CV events. Patients were followed-up for a mean of 3.7 years. The calculated rate of developing a first CV event during the study period was 1.11 events per 100 patient-years (95% CI 0.74–1.67). Higher burden of atherosclerotic plaques in the carotid arteries was associated with an increased risk of developing CV events even after adjusting for traditional CV risk factors. The authors suggested that combining vascular imaging data with information on traditional CV risk factors could improve the accuracy of CV risk stratification in patients with PsD and facilitate earlier initiation of appropriate treatment to reduce CV events [22].
Coronary CT Scans
The presence of a carotid artery plaque alone may not be sufficient to identify patients with inflammatory arthritis who are at risk for coronary artery disease (CAD) [23]. Coronary computed tomography angiography (CCTA) is a non-invasive technique that gives direct anatomic visualization of the coronary artery wall, reliably measures the overall plaque burden, size and severity of luminal restriction [24], and identifies vulnerable, lipid-rich plaques with a high likelihood of rupture leading to an acute coronary syndrome [25]. Despite that patients with PsD have an increased risk of CAD compared with controls [17, 26], there are limited data available on CAD as assessed by CCTA in this population. Hjuler and colleagues compared prevalence, severity, and subtypes of coronary plaques between patients with severe psoriasis, atopic dermatitis (AD), and asymptomatic controls without known CVD. They found that psoriasis patients had more proximal lesions, higher prevalence of significant stenosis, and three-vessel disease than patients with AD. Plaque burden was correlated with age, hyperlipidemia, and diabetes, but not with disease duration [27]. Increased prevalence, burden, and severity of coronary plaques was reported in PsA patients without prior diagnosis of CAD compared to healthy controls. PsA remained an independent factor for coronary plaques after adjusting for traditional CV risk factors and the presence of vulnerable plaques associated with age, male gender, and disease duration [28]. Our group showed that PsA patients without symptoms or diagnosis of CAD had a higher presence and extent of coronary plaques, particularly of vulnerable mixed plaque type, compared to controls. Interestingly, more PsA patients had plaques with higher plaque volume in the proximal left anterior descending coronary segments, which are known to be associated with a poorer prognosis of CAD. Independent predictors of increased plaque burden in PsA were age, maximum CRP, and number of swollen joints during the disease course, disease duration, and plasma glucose. Total plaque volume in the coronary arteries was associated with a diagnosis of PsA, but not with metabolic syndrome. Our results suggested that accelerated formation of coronary plaques in PsA may associate with underlying disease activity and severity but is independent of features of the metabolic syndrome [29]. A recent prospective observational study demonstrated a decrease in coronary artery plaque disease indices including lipid-rich plaque and necrotic core following treatment with biologic agents suggesting that treatment with biologics favorably modulates coronary artery plaques in psoriasis [30].
PET Scanning
18F-Fluorodeoxyglucose (FDG) imaging in positron emission tomography (PET) CT scanning is an advanced imaging technique that is exquisitely sensitive for detecting macrophage activity, and thus enables highly precise measurements of inflammation in atherosclerotic plaques both in large and medium-sized arteries. Vascular inflammation detected by FDG-PET/CT has been shown to predict CV events independent of traditional risk factors and is also highly associated with overall burden of atherosclerosis [31]. Moreover, vascular inflammation is highly sensitive to modulation of risk factors with preventive strategies such as statin therapy and therapeutic lifestyle changes, which are known to mitigate CV risk [32]. In a seminal study on 60 psoriasis patients using FDG-PET/CT, psoriasis severity was associated with aortic vascular inflammation beyond cardiovascular risk factors, suggesting shared mechanisms between remote inflammation in the skin and presence of vascular disease [33]. Associations between treatment of skin disease in psoriasis and change in aortic vascular inflammation by FDG-PET/CT at 1 year were investigated in a prospective cohort study including 115 patients with mild-to-moderate psoriasis, of whom 39% received systemic or biological treatment. Improvement in psoriasis was associated with improvement in aortic vascular inflammation independent of CV risk factors, with greater improvement in vascular inflammation observed in those who had higher than 75% reduction in skin disease severity. These findings suggest that alleviating remote skin inflammation may have positive effects on vascular inflammation at 1 year, similar to the effect of low-dose statin therapy [34]. Results from a recent prospective study on the effect of TNFi on subclinical CVD demonstrated reduced progression of carotid plaques by ultrasound in men with PsD and improvement in vascular inflammation by FDG-PET/CT in both men and women with PsA independently of traditional CV risk factors, indicating a potentially protective effect of targeted biologic treatments on CV risk in patients with PsD [35].
Risk Factors for Cardiovascular Disease in PsD
Very much in line with the data from psoriasis patients, PsD patients also have an increased prevalence of conventional cardiovascular risk factors compared to the normal population. Interestingly, PsD patients may also have an increased risk from non-conventional risk factors such as raised levels of homocysteine, increased burden of inflammation, and excessive alcohol consumption [36]. A few of the important comorbidities associated with PsD will be discussed here.
Hypertension
The prevalence of hypertension has been reported to be higher in patients with PsA compared to that in the general population [19, 37,38,39,40,41] and also in comparison to those patients with psoriasis only [42]. Overall, prevalence of hypertension is significantly higher in patients with PsD. For example, according to the results of a recent study, the chances of having uncontrolled blood pressure are increased in people with more severe psoriasis, and this association was still significant even after controlling for other hypertension risk factors such as high body mass index (BMI), diabetes mellitus (DM), and high cholesterol [43]. A meta-analysis confirmed an increased prevalence of hypertension in psoriasis patients, with odds ratios (OR) of 1.30 for mild psoriasis and 1.49 for severe psoriasis [44]. Interestingly, the presence of hypertension may increase the risk of incident psoriasis. In the Nurses’ Health Study involving 77,728 women, patients with hypertension were found to have a higher risk of developing psoriasis [45], possibly related to use of beta-blockers.
In a recent cross-sectional assessment of 283 PsA patients, we have shown that 74% had elevated blood pressure [46] with prevalence of hypertension in PsA significantly higher compared to the patients with non-inflammatory rheumatologic conditions (74 vs. 57%, p = 0.002) [47]. In another study, using a large population-based cohort (UK biobank), it has been shown that CVD outcomes, in particular hypertension, are significantly higher in PsA compared to psoriasis and is independent of known CVD risk factors [48].
Diabetes Mellitus
Potential association of PsD with DM was likely reported for the first time about six decades ago [49]. A recent meta-analysis (including about half of million patients with psoriasis and about 5 million controls), has shown significantly higher pooled OR for the association between psoriasis and the risk of type 2 DM (1.76, 95% CI 1.59–1.96), with a notable dose–effect of psoriasis on diabetes risk. Importantly, it was also observed that the risk of DM was highest among PsA patients compared to pooled OR (OR 2.18, 95% CI 1.36–3.50 vs. OR 1.76, 95% CI 1.59–1.96) [50]. Similarly, another population-based, age- and gender-matched study has revealed a possible association between PsA and diabetes, but only in women (OR 1.60, 95% CI 1.02–2.52, p = 0.040) [51]. In a recent study, it was noted that patients with PsA have a 43% higher risk of developing DM compared with the general population, and this risk was especially high in the younger age groups, and patients having higher levels of disease activity [52].
It remains to be seen that whether the drugs which effectively suppress the immune system reduce the risk of developing diabetes. However, one study compared large cohorts of RA and psoriasis patients and found that the use of a TNF inhibitor or hydroxychloroquine but not methotrexate was associated with a reduced risk of DM compared with other non-biologic DMARDs [53]. Further studies, especially in PsA patients, are needed to confirm these findings.
Obesity
BMI provides an easy way to measure obesity and is calculated as weight in kilograms divided by height in meters squared. Adipose tissue produces more than 50 cytokines and other molecules, which play an important role in the inflammatory cascade, and are known as adipokines. These adipokines include adiponectin, leptin, plasminogen activator inhibitor-1, IL-6, and TNFα [54]. Adipokines engage in a wide variety of physiological or pathological processes, including immunity and inflammation [55]. Hence, adipose tissue is now recognized as a pro-inflammatory state.
Being obese not only increases the burden of inflammation among patients with PsA, but it is also an established risk factor for the development of skin psoriasis. A number of case-controlled studies have shown that patients with skin psoriasis have higher BMI than the general population [56, 57]. In addition, such higher BMI patients are more likely to have more severe skin disease [58]. The potential link between obesity and PsA is even more complex; three large prospective studies have shown the dose-dependent link of obesity with the diagnosis of PsA even in different population settings [59,60,61].
Reassuringly, quite encouraging results are shown by interventional studies, where preventing or treating obesity was addressed. In a study by Di Minno et al., it was shown that losing 5–10% of total body weight was associated with a higher achievement of minimal disease activity (MDA) in PsA patients taking TNFi (OR 3.75, 95% CI 1.36–10.36, p = 0.011). Furthermore, those who lost more weight had even better achievement of MDA (OR 6.67, 95% CI 2.41–18.41, p < 0.001) [62].
A more recent prospective interventional study has found that obese patients with PsA who followed a short-term, very low-energy diet demonstrated improvements in multiple aspects of disease activity. Over a period of 6 months, patients on the restrictive diet (640 kcal/day) lost a median of 18.7 kg, which represented 18.6% of their weight, and the percentage of patients achieving MDA rose from 29.3% at baseline to 53.7% at 6 months (p = 0.002) [63].
Hyperlipidemia
Hypercholesterolemia is an established risk factor for CVDs. Interestingly, hypercholesterolemia, through the involvement of immune system, can induce inflammation in the vessel wall [64]. T-lymphocytes play an important role in PsA pathogenesis [65], and it has been sown that one of the early immune cell types activated by hypercholesterolemia is the T-lymphocyte [66].
In patients with psoriasis or PsA, several groups have found numerous lipid alterations, including changes in concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides, or lipoprotein Lp(a), or decreased concentrations of high-density lipoprotein cholesterol (HDL-C) [67, 68]. Moreover, patients with psoriasis or PsA have dysfunctional LDLs or HDLs, low-volume LDLs, and LDL efflux disturbances [69].
A recent important study has evaluated whether a history of hypercholesterolemia is associated with the risk of developing psoriasis and PsA in a cohort of 95,540 US women. The fully adjusted HRs of incident psoriasis and PsA associated with hypercholesterolemia were 1.25 (95% CI 1.04–1.50) and 1.58 (95% CI 1.13–2.23), respectively. Participants with hypercholesterolemia duration time ≥ 7 years were at a higher risk of developing psoriasis (HR = 1.29, 95% CI 1.03–1.61, Ptrend = 0.0002) and PsA (HR 1.68, 95% CI 1.12–2.52, Ptrend = 0.002). Importantly, this association was noted to be independent of cholesterol-lowering medication use [70].
Even more exciting has been the identification of specific immune cells, called CD1-restricted self-lipid–reactive T-cells in the blood or skin of humans [71], which may be the missing link between PsD and CVD. Furthermore, in a strain of mice with self-lipid–reactive T-cells and high levels of cholesterol, mice with hyperlipidemia began developing skin diseases mirroring the usual development of psoriasis in humans [72].
Metabolic Syndrome, Insulin Resistance, and the Role of Systemic Inflammation
Metabolic syndrome (MetSyn) is a cluster of five classic cardiovascular risk factors [73]. MetSyn is a well-recognized risk factor for CADs. Recent studies have estimated that the prevalence of MetSyn in the Western population is 15–24% [74, 75]; however, the prevalence of MetSyn among PsA patients has been reported to be much higher, at more than 40% [46].
It has been postulated that insulin resistance (IR) possibly explains the increased cardiovascular comorbidity associated with systemic inflammatory conditions, such as psoriasis and other immune-mediated inflammatory diseases [73]. IR in turn contributes to endothelial cell dysfunction, subsequently leading to atherosclerosis and finally to end organ damage, stroke or MI. There is compelling evidence that IR, MetSyn, and atherosclerotic events may well share a common inflammatory basis. There have been a number of studies showing high prevalence of MetSyn and IR among patients with PsA [46, 76, 77]. Additionally, we have also shown that MetSyn and IR are significantly associated with the severity of PsA after controlling for usual confounders, including severity of psoriasis [46].
Links between inflammation and cardiovascular comorbidities are increasingly recognized. Since in PsA there is a co-existence of skin and of musculoskeletal inflammation, there might be a greater burden of comorbidities, such as MetSyn and IR, and consequently of CVDs due to a greater inflammatory load [46]. There is emerging convincing data to support this concept with another study showing that an increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with PsA. The cumulative effect of inflammation was measured by the time-adjusted arithmetic mean of all measurements from the first visit to the clinic, which included inflammatory markers and disease activity indices for both psoriasis and PsA. However, these associations were not significant after adjustment for traditional cardiovascular risk factors, which suggested that the association might be partly mediated by traditional cardiovascular risk factors [17]. In a recent study, the cumulative inflammatory burden, as reflected by cumulative averages of repeated measures of ESR (ca-ESR), has been shown to associate with increased arterial stiffness in PsA patients even after adjustment for cardiovascular risk factors, emphasizing the important role of chronic inflammation in accelerating the development of cardiovascular risks in PsA [78].
There has been increasing recognition of arterial stiffness as a factor contributing to cardiovascular morbidity and mortality. Arterial pulse wave velocity (aPWV) has been used as the non-invasive standard for measuring arterial stiffness. In PsA, a significantly higher aPWV has been shown when compared to controls, even after controlling for age, weight, height, heart rate, central mean pressure, and other confounders. Importantly, an exclusion criterion in this study was the presence of known CV risk factors [79]. However, when endothelial progenitor cells were assessed as an essential regulatory element of the vascular system, this did not correlate with parameters of arterial stiffness [80]. Of note, is a recent study showing that achieving sustained MDA has a significant protective effect on vascular plaque progression (OR 0.273, 95% CI 0.088–0.846, p = 0.024) [81]. Finally, as referred to earlier, we have shown that higher coronary artery plaque burden in PsA is independent of MetSyn and is associated with underlying inflammatory disease severity [29]. We have also evaluated the performance of established CV risk scores against coronary artery plaque burden using CCTA in PsA patients without symptoms of CAD. It was noted that there is suboptimal performance of established cardiovascular risk scores, however, multiplication factor of 1.5 (to account for underlying inflammatory disease) accurately categorized PsA patients at "high-risk" of CVD, in line with the observations in RA [16].
Cardiovascular Disease Prevention in Patients with PsD
Increasing evidence points to good disease control as being important in mitigating the effects of systemic inflammation. The screening for and treatment of traditional risk factors such as hyperlipidemia or hypertension is also of significant importance but with some debate still occurring as to whom should be responsible for this aspect of patient care. There is a danger that with time at a premium in the busy doctor’s office that adequate attention to these risk factors will not be given and the patient will ultimately suffer as a result. The updated 2015–2016 EULAR recommendations for cardiovascular disease risk management in inflammatory musculoskeletal disease are quite clear as to who should take responsibility [82]. In their overarching principles, they state that it is the treating rheumatologist who is responsible and they should do so by ensuring that CVD risk assessment and management is being performed regularly, they should record who is performing it and should make sure that the patient is aware of the need for regular risk assessment. In patients with psoriasis, it is a little less clear as to who should take responsibility but guidelines do recommend that patients are screened for cardiovascular risk factors, and that patients are advised to stop smoking, reduce alcohol consumption, reduce weight, exercise three times a week for 30 min, and to have their blood pressure and cholesterol levels monitored and modified as necessary [83].
Future Studies
There is still much to learn about CVD in PsD, its causes, and prevention. In particular, we need to understand better the link between cardiovascular risk factors, cardiovascular events, and inflammation in PsD. Is the increased cardiovascular risk independent of traditional risk factors or is there an additive effect with persistent inflammatory disease? Understanding better the factors contributing to CVD will drive the rational development of risk prevention. In relation to biologic and small molecule therapy in PsD, further studies need to assess treatment benefit/risk ratio in relation to cardiovascular disease. Will more aggressive treatment targeting MDA or remission have a favorable effect or will such an approach potentially be associated with unanticipated adverse outcomes? Finally, patients need better education about cardiovascular risk and how to prevent it. We need to develop better techniques for implementing lifestyle changes and we need to understand whether treatment targets for blood pressure or for lipids are similar or are different for patients with PsD as compared to the general population.
Summary and Conclusions
Cardiovascular risk factors leading to accelerated atherosclerosis and cardiovascular events occur more commonly in patients with PsD with evidence accumulating that this is at least in part driven by persistent systemic inflammation. Further studies are required to better understand the mechanisms related to this risk so as to develop strategies aiming to improve cardiovascular related morbidity and mortality outcomes.
References
Toms TE, Symmons DP, Kitas GD. Dyslipidaemia in rheumatoid arthritis: the role of inflammation, drugs, lifestyle and genetic factors. Curr Vasc Pharmacol. 2010;8(3):301–26.
Arida A, Protogerou AD, Kitas GD, Sfikakis PP. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int J Mol Sci. 2018;19(7):1890.
Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39(4):787–98.
Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278(5):483–93.
Taleb S, Tedgui A. IL-17 in atherosclerosis: the good and the bad. Cardiovasc Res. 2018;114(1):7–9.
Eder L, Dey A, Joshi AA, Boehncke WH, Mehta NN, Szentpetery A. Cardiovascular diseases in psoriasis and psoriatic arthritis. J Rheumatol Suppl. 2019;95:20–7.
Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41.
Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–6.
Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Investig Dermatol. 2013;133(10):2340–6.
Jamnitski A, Symmons D, Peters MJ, Sattar N, McInnes I, Nurmohamed MT. Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis. 2013;72(2):211–6.
Eder L, Wu Y, Chandran V, Cook R, Gladman DD. Incidence and predictors for cardiovascular events in patients with psoriatic arthritis. Ann Rheum Dis. 2016;75(9):1680–6.
Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2017;69(1):67–74.
Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270(2):147–57.
Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–32.
Eder L, Chandran V, Gladman DD. The Framingham Risk Score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis. 2014;73(11):1990–6.
Haroon M, Szentpetery A, Dodd JD, Fitzgerald O. Modifications of cardiovascular risk scores, but not standard risk scores, improve identification of asymptomatic coronary artery disease in psoriatic arthritis. J Rheumatol. 2018;45(9):1329–30.
Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis. Ann Rheum Dis. 2015;74(10):1830–5.
Lucke M, Messner W, Kim ES, Husni ME. The impact of identifying carotid plaque on addressing cardiovascular risk in psoriatic arthritis. Arthritis Res Ther. 2016;18:178.
Kimhi O, Caspi D, Bornstein NM, Maharshak N, Gur A, Arbel Y, et al. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–9.
Balci DD, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009;23(1):1–6.
Eder L, Jayakar J, Shanmugarajah S, Thavaneswaran A, Pereira D, Chandran V, et al. The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis. 2013;72(5):715–20.
Sobchak C, Akhtari S, Harvey P, Gladman D, Chandran V, Cook R, et al. Value of carotid ultrasound in cardiovascular risk stratification in patients with psoriatic disease. Arthritis Rheumatol. 2019;71(10):1651–9.
Svanteson M, Rollefstad S, Klow NE, Hisdal J, Ikdahl E, Semb AG, et al. Associations between coronary and carotid artery atherosclerosis in patients with inflammatory joint diseases. RMD Open. 2017;3(2):e000544.
Carita P, Guaricci AI, Muscogiuri G, Carrabba N, Pontone G. Prognostic value and therapeutic perspectives of coronary CT angiography: a literature review. Biomed Res Int. 2018;2018:6528238.
Bom MJ, van der Heijden DJ, Kedhi E, van der Heyden J, Meuwissen M, Knaapen P, et al. Early detection and treatment of the vulnerable coronary plaque: can we prevent acute coronary syndromes? Circ Cardiovasc Imaging. 2017;10(5):e005973.
Kaiser H, Abdulla J, Henningsen KMA, Skov L, Hansen PR. Coronary artery disease assessed by computed tomography in patients with psoriasis: a systematic review and meta-analysis. Dermatology. 2019;235(6):478–87.
Hjuler KF, Bottcher M, Vestergaard C, Deleuran M, Raaby L, Botker HE, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. 2015;128(12):1325–34 e2.
Shen J, Wong KT, Cheng IT, Shang Q, Li EK, Wong P, et al. Increased prevalence of coronary plaque in patients with psoriatic arthritis without prior diagnosis of coronary artery disease. Ann Rheum Dis. 2017;76:1237–44.
Szentpetery A, Healy GM, Brady D, Haroon M, Gallagher P, Redmond CE, et al. Higher coronary plaque burden in psoriatic arthritis is independent of metabolic syndrome and associated with underlying disease severity. Arthritis Rheumatol. 2018;70(3):396–407.
Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–8.
Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A. Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). J Vis Exp. 2012;63:e3777.
Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17.
Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–76.
Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2(9):1013–8.
Eder L, Joshi AA, Dey AK, Cook R, Siegel EL, Gladman DD, et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol. 2018;70(3):408–16.
Zhu TY, Li EK, Tam LS. Cardiovascular risk in patients with psoriatic arthritis. Int J Rheumatol. 2012;2012:714321.
Kondratiouk S, Udaltsova N, Klatsky AL. Associations of psoriatic arthritis and cardiovascular conditions in a large population. Perm J. 2008;12(4):4–8.
Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol. 2011;30(7):877–85.
Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–72.
Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68(7):1131–5.
Tam LS, Tomlinson B, Chu TT, Li M, Leung YY, Kwok LW, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls–the role of inflammation. Rheumatology (Oxford). 2008;47(5):718–23.
Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken). 2011;63(12):1729–35.
Takeshita J, Wang S, Shin DB, Mehta NN, Kimmel SE, Margolis DJ, et al. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015;151(2):161–9.
Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31(3):433–42 (discussion 42–3).
Wu S, Han J, Li WQ, Qureshi AA. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol. 2014;150(9):957–63.
Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol. 2014;41(7):1357–65.
Haroon M, Rafiq Chaudhry AB, Fitzgerald O. Higher prevalence of metabolic syndrome in patients with psoriatic arthritis: a comparison with a control group of noninflammatory rheumatologic conditions. J Rheumatol. 2016;43(2):463–4.
Cook MJ, Bellou E, Bowes J, Sergeant JC, O’Neill TW, Barton A, et al. The prevalence of co-morbidities and their impact on physical activity in people with inflammatory rheumatic diseases compared with the general population: results from the UK Biobank. Rheumatology (Oxford). 2018;57(12):2172–82.
Brownstein MH. Psoriasis and diabetes mellitus. Arch Dermatol. 1966;93(6):654–5.
Coto-Segura P, Eiris-Salvado N, Gonzalez-Lara L, Queiro-Silva R, Martinez-Camblor P, Maldonado-Seral C, et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: a systematic review and meta-analysis. Br J Dermatol. 2013;169(4):783–93.
Dreiher J, Freud T, Cohen AD. Psoriatic arthritis and diabetes: a population-based cross-sectional study. Dermatol Res Pract. 2013;2013:580404.
Eder L, Chandran V, Cook R, Gladman DD. The risk of developing diabetes mellitus in patients with psoriatic arthritis: a cohort study. J Rheumatol. 2017;44(3):286–91.
Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–31.
Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29(1):81–90.
Otero M, Lago R, Gomez R, Lago F, Gomez-Reino JJ, Gualillo O. Leptin: a metabolic hormone that functions like a proinflammatory adipokine. Drug News Perspect. 2006;19(1):21–6.
Cohen AD, Gilutz H, Henkin Y, Zahger D, Shapiro J, Bonneh DY, et al. Psoriasis and the metabolic syndrome. Acta Derm Venereol. 2007;87(6):506–9.
Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216(2):152–5.
Murray ML, Bergstresser PR, Adams-Huet B, Cohen JB. Relationship of psoriasis severity to obesity using same-gender siblings as controls for obesity. Clin Exp Dermatol. 2009;34(2):140–4.
Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Duffin KC, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146(7):721–6.
Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71(8):1273–7.
Li W, Han J, Qureshi AA. Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis. 2012;71(8):1267–72.
Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014;73(6):1157–62.
Klingberg E, Bilberg A, Bjorkman S, Hedberg M, Jacobsson L, Forsblad-d’Elia H, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21(1):17.
Stokes KY, Calahan L, Hamric CM, Russell JM, Granger DN. CD40/CD40L contributes to hypercholesterolemia-induced microvascular inflammation. Am J Physiol Heart Circ Physiol. 2009;296(3):H689–97.
Haroon M, Fitzgerald O. Pathogenetic overview of psoriatic disease. J Rheumatol Suppl. 2012;89:7–10.
Stokes KY. Microvascular responses to hypercholesterolemia: the interactions between innate and adaptive immune responses. Antioxid Redox Signal. 2006;8(7–8):1141–51.
Jones SM, Harris CP, Lloyd J, Stirling CA, Reckless JP, McHugh NJ. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis. 2000;59(11):904–9.
Pietrzak A, Chodorowska G, Szepietowski J, Zalewska-Janowska A, Krasowska D, Hercogova J. Psoriasis and serum lipid abnormalities. Dermatol Ther. 2010;23(2):160–73.
He L, Qin S, Dang L, Song G, Yao S, Yang N, et al. Psoriasis decreases the anti-oxidation and anti-inflammation properties of high-density lipoprotein. Biochim Biophys Acta. 2014;1841(12):1709–15.
Wu S, Li WQ, Han J, Sun Q, Qureshi AA. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66(2):304–10.
Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV, et al. Human autoreactive T cells recognize CD1b and phospholipids. Proc Natl Acad Sci USA. 2016;113(2):380–5.
Bagchi S, He Y, Zhang H, Cao L, Van Rhijn I, Moody DB, et al. CD1b-autoreactive T cells contribute to hyperlipidemia-induced skin inflammation in mice. J Clin Investig. 2017;127(6):2339–52.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–9.
Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164(10):1066–76.
Raychaudhuri SK, Chatterjee S, Nguyen C, Kaur M, Jialal I, Raychaudhuri SP. Increased prevalence of the metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord. 2010;8(4):331–4.
Labitigan M, Bahce-Altuntas A, Kremer JM, Reed G, Greenberg JD, Jordan N, et al. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(4):600–7.
Shen J, Shang Q, Li EK, Leung YY, Kun EW, Kwok LW, et al. Cumulative inflammatory burden is independently associated with increased arterial stiffness in patients with psoriatic arthritis: a prospective study. Arthritis Res Ther. 2015;17:75.
Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010; 31(19):2338–50.
Patschan D, Sugiarto N, Henze E, Mossner R, Mohr J, Muller GA, et al. Early endothelial progenitor cells and vascular stiffness in psoriasis and psoriatic arthritis. Eur J Med Res. 2018;23(1):56.
Cheng IT, Shang Q, Li EK, Wong PC, Kun EW, Law MY, et al. Effect of achieving minimal disease activity on the progression of subclinical atherosclerosis and arterial stiffness: a prospective cohort study in psoriatic arthritis. Arthritis Rheumatol. 2019;71(2):271–80.
Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28.
Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17(11):1509–16.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Agnes Szentpetery has nothing to disclose. Muhammad Haroon has received educational grants from AbbVie and Pfizer, and has been a member of advisory boards for AbbVie and Celgene. Prof Oliver FitzGerald has received grants and/or honoraria from AbbVie, BMS, UCB, Pfizer, Lilly, Novartis, Janssen and Celgene.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.10265141.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Szentpetery, A., Haroon, M. & FitzGerald, O. Cardiovascular Comorbidities in Psoriatic Disease. Rheumatol Ther 7, 5–17 (2020). https://doi.org/10.1007/s40744-019-00185-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-019-00185-4