Abstract
Introduction
Controlled clinical studies have shown that the efficacy of tocilizumab (TCZ) monotherapy is superior to that of tumor necrosis factor inhibitor (TNFi) monotherapy and comparable to that of TCZ plus methotrexate (MTX) for the treatment of rheumatoid arthritis (RA). This study compared the real-world effectiveness of TCZ monotherapy vs. TNFis plus MTX in US patients with RA.
Methods
TCZ-naïve patients from the Corrona RA registry with prior exposure to ≥ 1 TNFi who initiated TCZ monotherapy or TNFi + MTX were included. Outcomes included mean change in Clinical Disease Activity Index (CDAI), achievement of low disease activity (LDA; CDAI ≤ 10), achievement of modified American College of Rheumatology (mACR) 20/50 responses, and mean change in modified Health Assessment Questionnaire (mHAQ) at 6 months. Patients initiating TNFi + MTX were grouped by MTX dose (≤ 10 mg; > 10 to ≤ 15 mg; > 15 to ≤ 20 mg; > 20 mg); outcomes in each group were compared with TCZ monotherapy using trimmed populations (excluding patients outside the propensity score distribution overlap).
Results
Patients in all groups experienced improvement in CDAI at 6 months (mean change, − 6.9 to − 9.7), with no significant differences between the TCZ monotherapy and TNFi + MTX groups. Achievement of LDA and mACR responses at 6 months were comparable between the TCZ monotherapy and TNFi + MTX groups; overall, 26.8–38.0% of patients achieved LDA, 24.3–37.6% achieved mACR20 response and 13.2–20.8% achieved mACR50 response. The mean change in mHAQ at 6 months was − 0.1 in all groups.
Conclusions
In this real-world population of US patients with RA who had prior TNFi exposure, there was no evidence of a difference in the effectiveness of TCZ monotherapy compared with that of TNFi + MTX, regardless of MTX dose, at 6 months for improving RA disease activity.
Funding
Corrona, LLC.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Plain Language Summary
Tumor necrosis factor inhibitors (TNFis) are often the first choice for biologic therapy in patients with rheumatoid arthritis (RA). However, up to 40% of patients may have an inadequate response to a TNFi; these patients may switch to a different TNFi or to a biologic with an alternative mechanism of action (MOA). Currently, limited and conflicting information is available regarding the benefits of switching to a biologic with an alternative MOA vs. a second TNFi. Comparative effectiveness studies of TNFis vs. non-TNFi biologics are needed to ensure the best clinical outcomes in patients with inadequate responses to TNFis.
Tocilizumab (TCZ) is an interleukin-6 receptor inhibitor approved for the treatment of moderate to severe RA. Whereas TNFis generally have increased efficacy when administered with methotrexate (MTX), TCZ has similar efficacy when administered as monotherapy or in combination with MTX. This study compared the effectiveness of TCZ monotherapy with that of TNFis plus varying dose ranges of MTX in patients with RA and prior treatment with 1 or more TNFi in routine US clinical practice. Patients treated with TCZ monotherapy or a TNFi with MTX experienced substantial and comparable improvement in RA disease activity at 6 months. There was no evidence of a difference in the effectiveness of TCZ monotherapy compared with TNFi with MTX, regardless of MTX dose, for improving RA disease activity in patients with prior TNFi exposure. These results can help guide decision-making when selecting a biologic therapy for patients with RA who were previously treated with TNFis.
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disorder characterized by joint inflammation that can result in pain, joint deformity, functional disability, and decreased health-related quality of life [1,2,3]. The goal of treatment in patients with RA is to reduce disease activity and improve patients’ clinical outcomes and quality of life. Current treatment guidelines recommend conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate (MTX), as first-line therapy, followed by addition of another csDMARD or a biologic therapy in patients with an inadequate response [4].
The first choice of biologic therapy is typically a tumor necrosis factor inhibitor (TNFi). Although TNFis are associated with improvement in the signs and symptoms of RA, 30–40% of patients may have an inadequate response to a TNFi due to primary lack of response or secondary treatment failure due to resistance or intolerance [5,6,7,8]. Patients with an inadequate response to a TNFi may switch to a different TNFi or to a biologic with an alternative mechanism of action (MOA) [4].
Limited and conflicting data are available regarding the benefits of switching to a biologic with an alternative MOA vs. a second TNFi in patients with RA. Observational studies conducted in Europe demonstrated that switching to rituximab was more effective than switching to a subsequent TNFi in patients with an inadequate response to 1 or more TNFi [9,10,11]. However, an observational study of US patients with RA showed similar improvement in disease activity between patients who switched to abatacept and those who switched to a subsequent TNFi [12]. In a multicenter, randomized controlled trial in France, a higher proportion of patients with an inadequate response to their first TNFi who switched to a biologic with a different MOA achieved low disease activity (LDA) at 24 and 52 weeks compared with those who switched to a second TNFi [13].
Tocilizumab (TCZ) is a monoclonal antibody that blocks the interleukin-6 receptor and is approved for the treatment of patients with moderate to severe RA who have had an inadequate response to 1 or more DMARD [14]. TCZ can be administered as monotherapy or in conjunction with MTX or other csDMARDs [14]. Whereas TNFis generally have increased efficacy when administered with concomitant MTX, clinical studies have shown that TCZ has similar efficacy when administered as monotherapy or in combination with csDMARDs [15, 16]. TCZ monotherapy may therefore be an effective treatment option for patients who cannot tolerate or prefer not to use MTX.
Currently, real-world studies comparing the effectiveness of TCZ monotherapy to TNFis + MTX in US patients with RA are lacking. The objective of this study was to compare the effectiveness of TCZ monotherapy with that of TNFis plus varying dose ranges of MTX in patients with RA and prior exposure to 1 or more TNFi in routine clinical practice in the United States.
Methods
Study Setting
The Corrona registry has been previously described in detail [17, 18]. Briefly, the Corrona RA registry (NCT01402661) is an independent, prospective observational cohort that collects longitudinal real-world data from patients and their treating rheumatologists [17, 19]. The registry includes patients recruited by 686 participating rheumatologists from 174 private and academic practice sites across 41 states in the United States. As of March 31, 2018, data on 48,535 patients with RA have been collected. Corrona’s database currently includes information from 367,457 patient visits and 169,968 patient-years of follow-up observation, with a mean duration of patient follow-up of 4.30 years (median, 3.35 years).
The study was conducted according to the current (2013) version of the Declaration of Helsinki. Ethics approvals for this study were obtained from a central institutional review board (New England Independent Review Board, IRB# 120160610). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs and documentation of approval was submitted to the Sponsor prior to initiating any study procedures.
Study Population and Data Collection
Eligible participants were TCZ-naïve patients with RA in the Corrona registry with prior use of ≥ 1 TNFi who initiated TCZ as monotherapy or a TNFi + MTX (without another csDMARD) between January 2010 and October 2015. Patients in LDA or remission as assessed by Clinical Disease Activity Index (CDAI; ≤ 10) were excluded. Patients must have had a follow-up visit 6 months after initiation with CDAI data available at baseline and follow-up; due to the observational nature of the study, the 6-month visit may have occurred in a window of 3–9 months. For patients with more than 1 visit within the 3- to 9-month window, the visit closest to 6 months was designated as the 6-month follow-up visit. For patients who initiated TCZ monotherapy or a TNFi + MTX between Corrona visits, demographic and clinical characteristics from the visit prior to initiation were used as baseline characteristics, provided the prior visit was ≤ 4 months before initiation. Patients who initiated a TNFi + MTX must have had MTX dose information available at baseline. If patients had more than 1 eligible initiation of TNFi + MTX, only the first initiation was included in the analysis. For patients who had an eligible TNFi + MTX initiation and an eligible TCZ initiation during the study period, only the TNFi + MTX initiation was included due to sample size considerations.
Data from Corrona were collected from physician and patient questionnaires completed during routine clinical encounters that occurred over the 6-month study period. Data recorded at the time of clinical encounter included use of csDMARDs and biologics; 28-joint tender and swollen counts; CDAI; modified Health Assessment Questionnaire (mHAQ); physician and patient assessments of global disease activity; patient assessment of pain; and American College of Rheumatology (ACR) functional status. Data on demographics, insurance status, comorbid conditions, physician and patient reported RA disease characteristics, and RA medications were available for ≥ 95% of patients.
Drug Exposure Cohorts
Patients who initiated a TNFi + MTX were grouped into four cohorts by weekly MTX dose: ≤ 10 mg; > 10 to ≤ 15 mg; > 15 to ≤ 20 mg; and > 20 mg. To balance for predisposing factors that may increase a patient’s likelihood of receiving either TCZ monotherapy or a TNFi + MTX, a propensity score (PS) [20, 21]—or the probability of treatment selection—was calculated for each eligible patient using baseline (at the time of drug initiation) patient demographics (age, sex, race, body mass index, smoking status, and work status), disease characteristics (duration of RA, ACR functional class, mHAQ, CDAI, and patient pain scores), and treatment history (prednisone use/dose and prior biologic use). Outcomes in patients receiving TCZ monotherapy and each TNFi + MTX group were compared in trimmed populations excluding patients outside the PS distribution overlap.
For sensitivity analyses, outcomes were compared between stratified-matched populations. Patients in the TCZ monotherapy group and each TNFi + MTX group were stratified by prior treatment with 1 vs. ≥ 2 prior TNFi and matched based on the PS using a caliper (maximum difference in propensity) of 0.02. The TCZ monotherapy group was matched separately with each TNFi + MTX group; i.e., the same group of patients receiving TCZ monotherapy were matched to each TNFi + MTX group.
Study Outcomes
The primary outcome was mean change from baseline in CDAI at 6 months. Secondary outcomes included the proportion of patients who achieved LDA (CDAI ≤ 10), the proportion of patients who achieved 20% or 50% improvement in modified ACR response criteria (mACR20 or mACR50, respectively) [22], and mean change from baseline in mHAQ at 6 months.
Statistical Analysis
All statistical analyses were performed using Stata version 14 (StataCorp LLC). Baseline characteristics were compared between the TCZ monotherapy and TNFi + MTX groups using standardized differences. Standardized differences provide a measure of clinically important differences, even if there are no statistically significant differences. A standardized difference < 0.1 has been taken to indicate a negligible difference in the mean or prevalence of a covariate between treatment groups [20].
Patients were included regardless of switching or discontinuation of the initial biologic. For patients who discontinued their initial therapy prior to the 6-month follow-up visit but did not switch to another biologic, the outcomes at the 6-month visit were used for analyses. For patients who switched biologics prior to the 6-month follow-up visit, continuous outcomes (mean changes in CDAI and mHAQ) were assessed using last observation on drug carried forward and binary outcomes (achievement of LDA and mACR 20/50 responses) were assessed by nonresponse imputation.
Outcomes were analyzed in the trimmed populations by random effects (adjusted for clustering by site) linear models for continuous outcomes and random effects logistic regression models for binary outcomes. For continuous outcomes, the effect of TCZ monotherapy vs. each TNFi + MTX dose group was assessed by an estimated beta coefficient from the linear regression model representing the difference in mean outcomes between the groups. For binary outcomes, the effect of TCZ monotherapy vs. each TNFi + MTX dose group was assessed by an estimated odds ratio (OR) representing the increase or decrease in odds of the outcome. In multivariable adjusted models, outcomes were analyzed using multivariable models adjusting for the covariates included in the PS to account for remaining imbalances among the trimmed populations; 95% confidence intervals were estimated for each effect. For the matched analyses, estimated differences or odds ratios were calculated adjusting for clustering on the matched pairs with 95% confidence intervals for each effect. As an additional sensitivity analysis, outcomes in the matched pairs were analyzed using multivariable adjusted models with 95% confidence intervals for each effect.
Results
Patient Demographics and Baseline Clinical Characteristics
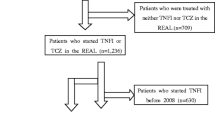
A total of 301 patients who initiated TCZ monotherapy and 772 patients who initiated a TNFi + MTX met the inclusion criteria prior to implementation of the PS (Fig. 1). Among the TNFi + MTX initiators, 116 initiated with MTX ≤ 10 mg, 200 with MTX > 10 to ≤ 15 mg, 337 with MTX > 15 to ≤ 20 mg and 119 with MTX > 20 mg. Among the patients treated with TCZ monotherapy, 96% were receiving TCZ intravenously every 4 weeks; of the 4% of patients (n = 12) receiving TCZ subcutaneously, seven patients received doses every 2 weeks, three received doses every week, and two had missing dose information. Patient demographics and baseline characteristics of the untrimmed population are described in Supplementary Table 1.
Patient disposition. aThe 6-month follow-up visit occurred in a window of 3–9 months. bOne initiation per patient across both groups, with preference for keeping TNFi initiation if feasible. CDAI, clinical disease activity index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; MTX, methotrexate; TCZ, tocilizumab; TNFi, tumor necrosis factor inhibitor
After excluding patients outside the PS distribution overlap and restricting the data to unique initiations per patient, the four trimmed cohorts included 283 TCZ monotherapy initiators vs. 108 TNFi + MTX ≤ 10 mg; 300 TCZ monotherapy initiators vs. 186 TNFi + MTX > 10 to ≤ 15 mg; 292 TCZ monotherapy initiators vs. 273 TNFi + MTX > 15 to ≤ 20 mg; and 285 TCZ monotherapy initiators vs. 107 TNFi + MTX > 20 mg. The stratified-matched populations included 61 patients in each arm receiving TCZ monotherapy vs. TNFi + MTX ≤ 10 mg; 71 receiving TCZ monotherapy vs. TNFi + MTX > 10 to ≤ 15 mg; 95 receiving TCZ monotherapy vs. TNFi + MTX > 15 to ≤ 20 mg; and 53 receiving TCZ monotherapy vs. TNFi + MTX > 20 mg.
Patient demographics and baseline characteristics were generally comparable between the TCZ monotherapy and TNFi + MTX groups in the trimmed cohorts (Table 1). Patients receiving TCZ monotherapy had significantly longer disease duration than those who received TNFi with one of the higher MTX doses (> 15 to ≤ 20 mg and > 20 mg). Higher proportions of patients receiving TCZ monotherapy had received ≥ 3 prior biologics and ≥ 2 prior TNFi than patients in all the TNFi + MTX dose groups. There were no significant differences in patient demographics, baseline clinical characteristics, or treatment history between the TCZ monotherapy and TNFi + MTX groups in the stratified-matched populations (Supplementary Table 2).
In the trimmed population, 70.0–71.6% of patients in the TCZ monotherapy groups and 64.8–73.7% of patients in the TNFi + MTX groups were still receiving their initial biologic at 6 months; 11.6–12.7% of patients in the TCZ monotherapy groups and 9.7–16.7% of patients in the TNFi + MTX groups switched to a new biologic, and 16.8–17.3% and 15.7–18.0%, respectively, discontinued without switching (Table 2). In the stratified-matched population, 63.9–77.4% of patients in the TCZ monotherapy groups and 59.0–68.9% of patients in the TNFi + MTX groups were still receiving their initial biologic at 6 months; 9.4–21.3% of patients in the TCZ monotherapy groups and 11.3–16.8% of patients in the TNFi + MTX groups switched to a new biologic, and 8.5–16.8% and 18.9–24.2%, respectively, discontinued without switching (Table 2). No significant differences were observed between the TCZ monotherapy and TNFi + MTX groups in the rates of switching or discontinuation of the initial biologic over the 6-month study period (Table 2).
Outcomes at 6 Months
All treatment groups experienced improvement from baseline in CDAI at 6 months (Supplementary Table 3). Mean change from baseline in CDAI ranged from − 6.9 to − 9.7 in the trimmed population and from − 6.1 to − 9.9 in the stratified-matched population. In unadjusted analyses in the trimmed population, there were no significant differences in change in CDAI at 6 months between patients who received TCZ monotherapy and those who received TNFi + MTX, except MTX > 15 to ≤ 20 mg; however, there were no significant differences after adjustment for covariates used in the PS (Fig. 2) and no differences in unadjusted or adjusted analyses of the stratified-matched population (Fig. 2; Supplementary Fig. 1).
Differences in mean change from baseline in CDAI at 6 months, TCZ monotherapy vs. TNFi + MTX. aAdjusted for age, sex, race (white vs. nonwhite), disabled, retired, baseline CDAI, baseline modified Health Assessment Questionnaire, baseline patient pain, baseline prednisone use/dose, baseline body mass index, prior biologic use, prior TNFi use, and American College of Rheumatology functional class. CDAI, Clinical Disease Activity Index; mono, monotherapy; MTX, methotrexate; TCZ, tocilizumab; TNFi, tumor necrosis factor inhibitor
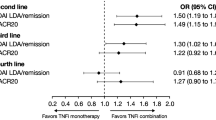
Overall, 26.8–38.0% of patients in the trimmed population and 20.8–38.0% in the stratified-matched population achieved LDA at 6 months (Supplementary Table 3). There were no significant differences in the likelihood of achieving LDA at 6 months between patients receiving TCZ monotherapy and any of the TNFi + MTX groups in unadjusted or adjusted analyses of the trimmed or stratified-matched populations (Fig. 3; Supplementary Fig. 2).
ORs for achievement of LDA (CDAI ≤ 10) at 6 months, TCZ monotherapy vs. TNFi + MTX. aAdjusted for age, sex, race (white vs. nonwhite), disabled, retired, baseline CDAI, baseline modified Health Assessment Questionnaire, baseline patient pain, baseline prednisone use/dose, baseline body mass index, prior biologic use, prior TNFi use, and American College of Rheumatology functional class. CDAI, Clinical Disease Activity Index; LDA, low disease activity; mono, monotherapy; MTX, methotrexate; OR, odds ratio; TCZ, tocilizumab; TNFi, tumor necrosis factor inhibitor
The proportions of patients with mACR20 response at 6 months ranged from 24.3 to 37.6% in the trimmed population and 22.1 to 37.1% in the stratified-matched population (Supplementary Table 3). The proportions of patients with mACR50 response at 6 months ranged from 13.2 to 20.8% in the trimmed population and 11.6 to 19.7% in the stratified-matched population (Supplementary Table 3). In the trimmed population, there were no significant differences in the likelihood of achieving mACR20 or mACR50 responses at 6 months between patients receiving TCZ monotherapy and those receiving TNFi + MTX in unadjusted or adjusted analyses, except MTX > 15 to ≤ 20 mg (Fig. 4). In the stratified-matched population, there were no significant differences in the likelihood of achieving mACR20 or mACR50 response between the TCZ monotherapy and any TNFi + MTX group in unadjusted or adjusted analyses (Fig. 4; Supplementary Fig. 3).
ORs for achievement of mACR20 and mACR50 responses at 6 months, TCZ monotherapy vs. TNFi + MTX. aAdjusted for age, sex, race (white vs. nonwhite), disabled, retired, baseline Clinical Disease Activity Index, baseline modified Health Assessment Questionnaire, baseline patient pain, baseline prednisone use/dose, baseline body mass index, prior biologic use, prior TNFi use, and ACR functional class. mACR, modified American College of Rheumatology response; mono, monotherapy; MTX, methotrexate; OR, odds ratio; TCZ, tocilizumab; TNFi, tumor necrosis factor inhibitor
In the trimmed population, the mean change from baseline in mHAQ at 6 months was − 0.1 in all treatment groups; in the stratified-matched population, the mean change from baseline in mHAQ ranged from 0 to − 0.2 (Supplementary Table 3). There were no significant differences in the mean change from baseline in mHAQ at 6 months between the TCZ monotherapy and TNFi + MTX treatment groups in unadjusted or adjusted analyses of the trimmed or stratified-matched populations (Fig. 5; Supplementary Fig. 4).
Differences in mean change from baseline in mHAQ at 6 months, TCZ monotherapy vs. TNFi + MTX. aAdjusted for age, sex, race (white vs. nonwhite), disabled, retired, baseline Clinical Disease Activity Index, baseline mHAQ, baseline patient pain, baseline prednisone use/dose, baseline body mass index, prior biologic use, prior TNFi use, and American College of Rheumatology functional class. mHAQ, modified Health Assessment Questionnaire; mono, monotherapy; MTX, methotrexate; TCZ, tocilizumab; TNFi, tumor necrosis factor inhibitor
Discussion
Using data from the US-based Corrona RA registry, we compared the clinical effectiveness of TCZ monotherapy with that of TNFis with varying dose ranges of MTX in patients with moderate to high disease activity and prior TNFi exposure seen in routine clinical practice. Patients who initiated a TNFi + MTX were grouped into four cohorts by weekly MTX dose: ≤ 10 mg; > 10 to ≤ 15 mg; > 15 to ≤ 20 mg; and > 20 mg. To minimize selection bias, study outcomes were compared between patients receiving TCZ monotherapy and each TNFi + MTX group in trimmed populations excluding patients outside the PS distribution overlap and in stratified-matched populations in which patients were stratified by the number of prior TNFi and matched based on the PS. Treatment with either TCZ monotherapy or a TNFi + MTX was associated with improvement in RA disease activity, and outcomes were comparable between patients who initiated TCZ monotherapy and those who initiated a TNFi + MTX, regardless of MTX dose, over 6 months. In adjusted analyses of both the trimmed and stratified-matched populations, there were no significant differences in the improvement of CDAI or mHAQ or the likelihood of achieving LDA (CDAI ≤ 10) or mACR responses at 6 months between the TCZ monotherapy and TNFi + MTX groups.
Randomized clinical trials have demonstrated that TCZ monotherapy is more effective than MTX monotherapy or monotherapy with the TNFi adalimumab for the improvement of RA disease activity [23, 24]. However, there are limited data available regarding the effectiveness of TCZ monotherapy compared with TNFi + MTX. Additionally, these trials excluded patients with prior biologic exposure or patients with an inadequate response to a prior TNFi; comparative effectiveness studies of TCZ monotherapy vs. TNFi + MTX in patients with prior biologic experience are lacking.
A recent analysis from the TOCERRA collaboration of European registries compared the effectiveness of TCZ vs. TNFis as monotherapy or in combination with csDMARDs in biologic-experienced patients with RA [25]. No significant differences were observed in change in CDAI score at 1 year among patients who initiated TNFi monotherapy (mean change, − 3.54), TNFi + csDMARDs (− 3.34), TCZ monotherapy (− 3.58) or TCZ + csDMARDs (− 3.68) [25]. In addition, no significant differences were observed in the proportion of patients who achieved CDAI LDA at 1 year among the treatment groups [25]. To our knowledge, ours is the first US study to evaluate the comparative effectiveness of switching to a TNFi with varying dose ranges of MTX vs. switching to monotherapy with a biologic with a different MOA after an inadequate response to a TNFi in a population of patients seen in routine clinical practice. Consistent with the results from TOCERRA, treatment with TCZ monotherapy or a TNFi + MTX resulted in comparable changes in CDAI scores and rates of achievement of LDA at 6 months in our US study population. Our study also found no evidence of a difference in the effectiveness of TCZ monotherapy or a TNFi + MTX with respect to improvement in mHAQ and achievement of mACR20/50 responses. Thus, our findings support those from the TOCERRA registry study and provide further evidence for the comparable effectiveness of TCZ monotherapy with that of TNFi + MTX in patients previously treated with biologics.
We evaluated TNFis administered with various dose ranges of MTX and observed comparable disease outcomes at 6 months in patients treated with TCZ monotherapy vs. those treated with a TNFi + MTX, regardless of MTX dose. Comparable effectiveness of increasing MTX doses in combination with TNFis has been observed in randomized clinical trials. The MUSICA trial assessed disease outcomes in patients with RA treated with adalimumab plus MTX 7.5 mg/week or 20 mg/week; no significant differences were observed in the proportions of patients who achieved ACR20/50/70 responses or CDAI LDA at week 24 between the MTX dose groups [26]. Similarly, a pooled analysis of the TEMPO and COMET trials found no significant differences in the rates of DAS28 LDA and remission and ACR20/50/70 responses over 6, 12, and 24 months among biologic-naïve patients treated with etanercept plus MTX < 10 mg/week, 10–17.5 mg/week, or > 17.5 mg/week [27]. Additionally, results from the TOCERRA registry study showed similar rates of CDAI remission and LDA between patients who received TCZ monotherapy and patients who received a TNFi with MTX across MTX doses [25]. The comparable effectiveness of TCZ monotherapy vs. TNFis + MTX across various MTX dose ranges observed in our study reflect the observations of these previous studies.
The results of this study have important clinical relevance to practicing rheumatologists. Comparative effectiveness studies of TNFis vs. non-TNFi biologics are necessary to help guide decision-making when selecting a biologic therapy for patients with prior TNFi exposure. Additionally, comparative effectiveness studies of TNFis + MTX vs. monotherapy with biologics with different MOAs are needed to ensure the best clinical outcomes in patients for whom MTX use is contraindicated. In the absence of head-to-head clinical trials, observational studies can provide important information for clinicians when deciding which biologic to prescribe a patient with prior TNFi exposure. This study showed that TCZ monotherapy and TNFi + MTX were associated with substantial and comparable clinical improvement in real-world clinical practice. Thus, factors such as individual patient characteristics, pharmacy benefit plans, and patient preferences will likely inform individual biologic prescription choices.
This study has several strengths. This all-comers study design recruited participants from multiple rheumatology centers across the United States, resulting in a range of patients with real-world disease activity and comorbidities not typically seen in randomized controlled trials. The prospective, observational nature of this study allowed us to compare the efficacy of TCZ monotherapy with that of TNFi + MTX in routine management of RA. Patients initiating a TNFi were stratified by concomitant MTX dose to account for the effect of MTX on disease activity outcomes. Patients were included in the analyses regardless of switching or discontinuing their initial biologic, which allowed a more conservative estimate of effectiveness [28]. Comparative effectiveness was broadly examined using multiple assessments, including CDAI, mACR response, and mHAQ.
This study also has some limitations. A general limitation of real-world observational studies is the concern that patients enrolled in registries may not be representative of patients observed elsewhere in general practice. However, a previous study found that Medicare patients in the Corrona registry were similar to the national US Medicare RA population in terms of demographic characteristics and comorbid conditions, suggesting that data from patients in Corrona may be generalizable to the population of patients with RA in the United States [19]. Another general limitation of real-world observational studies is the potential for bias because physicians prescribe therapies based on a patient’s profile and treatment selection is not random. To address this limitation, analyses were conducted in two populations: a trimmed population excluding patients outside the PS overlap (not on common support) and a population of patients stratified by prior TNFi use and matched on PS. However, this method does not address unmeasured confounding variables. Reasons for discontinuation of prior TNFis were not available for all patients; thus, sensitivity analyses were not performed based on reasons for discontinuation of prior TNFis, although other studies have shown that this may influence treatment response [29]. For example, it is possible that the occurrence of a serious adverse event may influence the likelihood of switching from a TNFi to a biologic with a different MOA. Additionally, small sample sizes prohibited the evaluation of individual TNFis; rather, TNFis were pooled and analyzed as a group. Thus, no conclusions can be drawn regarding differences in the effectiveness of TCZ monotherapy vs. specific TNFis + MTX. Choice of specific TNFi and patients’ treatment history may affect the impact of MTX dose on disease outcomes in patients treated with TNFi + MTX. Finally, follow-up time was limited to 6 months; longer follow-up is necessary to assess the long-term effectiveness of switching from a TNFi to TCZ monotherapy vs. a subsequent TNFi + MTX.
Conclusions
This observational, real-world study of US patients with RA showed no evidence of a difference in the efficacy of TCZ monotherapy compared with TNFi + MTX, regardless of MTX dose, for improving RA disease activity in patients with prior TNFi exposure. There was no evidence of differences in clinical outcomes at 6 months, including improvement in CDAI, achievement of LDA, achievement of mACR20 or mACR50 response, and improvement in mHAQ, between patients who switched to TCZ monotherapy and those who switched to a subsequent TNFi + MTX. These results suggest that, in clinical practice, TCZ monotherapy may be an effective treatment option for patients with RA who have been previously treated with TNFis.
References
Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906.
Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:943–67.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:1–25.
Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind, randomised controlled trial. Lancet. 2004;363:675–81.
Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343:1594–602.
Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT study group. Lancet. 1999;354:1932–9.
Finckh A, Ciurea A, Brulhart L, et al. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum. 2007;56:1417–23.
Gomez-Reino JJ, Maneiro JR, Ruiz J, et al. Comparative effectiveness of switching to alternative tumour necrosis factor (TNF) antagonists versus switching to rituximab in patients with rheumatoid arthritis who failed previous TNF antagonists: the MIRAR study. Ann Rheum Dis. 2012;71:1861–4.
Kekow J, Mueller-Ladner U, Schulze-Koops H. Rituximab is more effective than second anti-TNF therapy in rheumatoid arthritis patients and previous TNFalpha blocker failure. Biologics. 2012;6:191–9.
Harrold LR, Reed GW, Kremer JM, et al. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis. 2015;74:430–6.
Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs. a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316:1172–80.
Actemra (tocilizumab) [package insert]. South San Francisco: Genentech, Inc; 2016.
Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1897–904.
Gabay C, Hasler P, Kyburz D, et al. Biological agents in monotherapy for the treatment of rheumatoid arthritis. Swiss Med Wkly. 2014;144:w13950.
Kremer JM. The CORRONA database. Clin Exp Rheumatol. 2005;23:S172–7.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(Suppl 10):96–9.
Curtis JR, Chen L, Bharat A, et al. Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken). 2014;66:1790–8.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Goldman JA, Xia HA, White B, Paulus H. Evaluation of a modified ACR20 scoring system in patients with rheumatoid arthritis receiving treatment with etanercept. Ann Rheum Dis. 2006;65:1649–52.
Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50.
Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96.
Lauper K, Nordstrom DC, Pavelka K, et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann Rheum Dis. 2018;77:1276–82.
Kaeley GS, Evangelisto AM, Nishio MJ, et al. Methotrexate dosage reduction upon adalimumab initiation: clinical and ultrasonographic outcomes from the randomized noninferiority MUSICA trial. J Rheumatol. 2016;43:1480–9.
Gallo G, Brock F, Kerkmann U, Kola B, Huizinga TW. Efficacy of etanercept in combination with methotrexate in moderate-to-severe rheumatoid arthritis is not dependent on methotrexate dosage. RMD Open. 2016;2:e000186.
Harrold L, Reed G, Magner R, et al. Comparative effectiveness of rituximab versus anti-tumor necrosis factor switching for rheumatoid arthritis. Ann Rheum Dis. 2013;72:460.
Finckh A, Ciurea A, Brulhart L, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010;69:387–93.
Acknowledgements
Funding
This study is sponsored by Corrona, LLC. Corrona, LLC has been supported through contracted subscriptions in the last two years by AbbVie, Amgen, Bristol-Myers Squibb, Crescendo, Eli Lilly and Company, Genentech, GSK, Horizon Pharma USA, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer, Roche, and UCB. The study design and conduct were the result of a collaborative effort between Corrona, LLC and Genentech, Inc., and financial support for the study was provided by Genentech, Inc. Genentech, Inc. participated in the interpretation of data, review, and approval of the manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Article processing charges were funded by F. Hoffmann-LaRoche, Ltd.
Medical Writing and Editorial Assistance
Support for third-party writing assistance for this manuscript, furnished by Elizabeth Ohneck, PhD, of Health Interactions, Inc., was provided by F. Hoffmann-La Roche, Ltd/Genentech, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
L. R. Harrold is an employee of University of Massachusetts Medical School and Corrona, LLC, and shareholder of Corrona, LLC, and has received research support from Pfizer. G. W. Reed is an employee of University of Massachusetts Medical School and Corrona, LLC, and shareholder of Corrona, LLC. J. Best is an employee of Genentech, Inc. S. Zlotnick is an employee of Genentech, Inc. J. M. Kremer is an employee and shareholder of Corrona, LLC, is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Inc., GSK, Lilly, Pfizer, Regeneron, and Sanofi, and has received research support from AbbVie, Genentech, Inc., Lilly, Novartis, and Pfizer.
Compliance with Ethics Guidelines
Ethics approvals for this study were obtained from a central institutional review board (New England Independent Review Board, IRB# 120160610). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs and documentation of approval was submitted to the sponsor prior to initiating any study procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7106438.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Harrold, L.R., Reed, G.W., Best, J. et al. Real-world Comparative Effectiveness of Tocilizumab Monotherapy vs. Tumor Necrosis Factor Inhibitors with Methotrexate in Patients with Rheumatoid Arthritis. Rheumatol Ther 5, 507–523 (2018). https://doi.org/10.1007/s40744-018-0127-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0127-1