Abstract
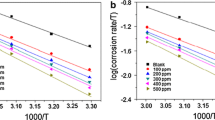
The study of the inhibition of the ecological corrosion of steel in the 1M hydrochloric acid medium by aqueous and ethanolic extracts of Pelargonium graveolens was carried out using gravimetric, electrochemical, high-performance liquid chromatography (HPLC), and scanning electron microscopy (SEM/EDX). The results of this study showed that inhibitory efficacy (IE) increases with increased concentration. It reaches a maximum value of 97% for the aqueous extract of P. graveolens and 92% for the ethanolic extraction for the concentrate of 1.0 g L−1 and decreases with the increase in temperature. The study of the influence of temperature has made it possible to understand the mechanism of action of these inhibitors on the corrosion of the steel so that the active molecules of the studied extracts are fixed on the metallic surface by forming physical bonds according to the adsorption isotherm Langmuir. These extracts behave as mixed-type inhibitors. These results are confirmed by a study of density functional theory (DFT) using the B3LYP/6-31G(d,p).












Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
C EA (2015) Corrosion engineering principles and solved problems—Branko N. Popov. ISBN 978-0-444-62722-3
Souto RM, Ait Albrimi Y, Ait Addi A et al (2016) Studies on the adsorption of heptamolybdate ions on AISI 304 stainless steel from acidic HCl solution for corrosion inhibition. Int J Electrochem Sci 11(1):385–397
Onukwuli OD, Anadebe VC, Nnaji PC et al (2021) Effect of pigeon pea seed (isoflavone) molecules on corrosion inhibition of mild steel in oilfield descaling solution: electro-kinetic, DFT modeling and optimization studies. J Iran Chem Soc 18:2983–3005. https://doi.org/10.1007/s13738-021-02250-8
Zhang M, Guo L, Zhu M et al (2021) Akebia trifoliate koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: integrated experimental and theoretical investigations. J Ind Eng Chem 101:227–236. https://doi.org/10.1016/j.jiec.2021.06.009
Majd MT, Ramezanzadeh M, Ramezanzadeh B, Bahlakeh G (2020) Production of an environmentally stable anti-corrosion film based on Esfand seed extract molecules-metal cations: integrated experimental and computer modeling approaches. J Hazard Mater 382:121029. https://doi.org/10.1016/j.jhazmat.2019.121029
Hameed RSA, Essa A, Nassar A et al (2022) Chemical and electrochemical studies on expired lioresal drugs as corrosion inhibitors for carbon steel in sulfuric acid. J New Mater Electrochem Syst 25:268–276. https://doi.org/10.14447/jnmes.v25i4.a07
Saady A, Ech-chihbi E, El-Hajjaji F et al (2021) Molecular dynamics, DFT and electrochemical to study the interfacial adsorption behavior of new imidazo[4,5-b] pyridine derivative as a corrosion inhibitor in acid medium. J Appl Electrochem 51:245–265. https://doi.org/10.1007/s10800-020-01498-x
Satapathy AK, Gunasekaran G, Sahoo SC et al (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856. https://doi.org/10.1016/j.corsci.2009.08.016
Mejeha IM, Nwandu MC, Okeoma KB et al (2012) Experimental and theoretical assessment of the inhibiting action of Aspilia africana extract on corrosion aluminum alloy AA3003 in hydrochloric acid. J Mater Sci 47(6):2559–2572. https://doi.org/10.1007/s10853-011-6079-2
Anadebe VC, Nnaji PC, Okafor NA et al (2021) Evaluation of bitter kola leaf extract as an anticorrosion additive for mild steel in 1.2 M H2SO4 electrolyte. S Afr J Chem 75:6–17
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415. https://doi.org/10.1016/j.corsci.2011.11.005
Nnanna LA, Onwuagba BN, Mejeha IM, Okeoma KB (2010) Inhibition effects of some plant extracts on the acid corrosion of aluminum alloy. Afr J Pure Appl Chem 4:011–016. https://doi.org/10.5897/AJPAC.9000079
Moretti G, Guidi F, Grion G (2004) Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros Sci 46:387–403. https://doi.org/10.1016/S0010-938X(03)00150-1
Fallavena T, Antonow M, Gonçalves RS (2006) Caffeine as non-toxic corrosion inhibitor for copper in aqueous solutions of potassium nitrate. Appl Surf Sci 2:566–571. https://doi.org/10.1016/j.apsusc.2005.12.114
Torres VV, Amado RS, de Sá CF et al (2011) Inhibitory action of aqueous coffee ground extracts on the corrosion of carbon steel in HCl solution. Corros Sci 53:2385–2392. https://doi.org/10.1016/j.corsci.2011.03.021
Riffi O, Salim R, Ech-chihbi E et al (2022) Experimental and quantum studies of Dysphania ambrosioïdes (L.) as ecological corrosion inhibitor for mild steel in hydrochloric acid environment. J Bio- Tribo-Corros 8:113. https://doi.org/10.1007/s40735-022-00712-x
Omar H, Elsayed T, El-Houda N et al (2020) Gene-targeted molecular phylogeny, phytochemical analysis, antibacterial and antifungal activities of some medicinal plant species cultivated in Egypt. Phytochem Anal. https://doi.org/10.1002/pca.3018
Bhardwaj N, Sharma P, Kumar V (2021) Corrosion inhibition property and adsorption behavior of P. tremula leaf extract in acidic media for steel used in petroleum industry (SS-410). Prot Met Phys Chem Surf 57:1076–1084. https://doi.org/10.1134/S2070205121050051
Bhardwaj N, Sharma P, Singh K et al (2021) Phyllanthus emblica seed extract as corrosion inhibitor for stainless steel used in the petroleum industry (SS-410) in acidic medium. Chem Phys Impact 3:100038. https://doi.org/10.1016/j.chphi.2021.100038
Yadav M, Behera D, Kumar S, Sinha RR (2013) Experimental and quantum chemical studies on the corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl. Ind Eng Chem Res 52:6318–6328. https://doi.org/10.1021/ie400099q
Zerga B, Sfaira M, Taleb M et al (2012) Adsorption and corrosion inhibition of some tripodal compounds for mild steel in molar hydrochloric acid medium. Pharma Chem 4:1887–1896
Marsoul A, Ijjaali M, Elhajjaji F et al (2020) Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L.): evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater Today Proc 27:3193–3198. https://doi.org/10.1016/j.matpr.2020.04.202
El-Hajjaji F, Merimi I, Messali M et al (2019) Experimental and quantum studies of newly synthesized pyridazinium derivatives on mild steel in hydrochloric acid medium. Mater Today Proc 13:822–831. https://doi.org/10.1016/j.matpr.2019.04.045
El-Hajjaji F, Ech-chihbi E, Rezki N et al (2020) Electrochemical and theoretical insights on the adsorption and corrosion inhibition of novel pyridinium-derived ionic liquids for mild steel in 1 M HCl. J Mol Liq 314:3737. https://doi.org/10.1016/j.molliq.2020.113737
Saady A, El-Hajjaji F, Taleb M et al (2018) Experimental and theoretical tools for corrosion inhibition study of mild steel in aqueous hydrochloric acid solution by new indanones derivatives. Mater Discov 12:30–42. https://doi.org/10.1016/j.md.2018.11.001
Anadebe VC, Nnaji PC, Onukwuli OD et al (2022) Multidimensional insight into the corrosion inhibition of salbutamol drug molecule on mild steel in oilfield acidizing fluid: Experimental and computer-aided modeling approach. J Mol Liq 349:118482. https://doi.org/10.1016/j.molliq.2022.118482
Anadebe VC, Chukwuike VI, Selvaraj V et al (2022) Sulfur-doped graphitic carbon nitride (S-g-C3N4) as an efficient corrosion inhibitor for X65 pipeline steel in CO2- saturated 3.5% NaCl solution: electrochemical, XPS and nanoindentation studies. Process Saf Environ Prot 164:715–728. https://doi.org/10.1016/j.psep.2022.06.055
Tang Y, Zhang F, Hu S et al (2013) Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: gravimetric, electrochemical, SEM, and XPS studies. Corros Sci Complete. https://doi.org/10.1016/j.corsci.2013.04.053
Haque J, Srivastava V, Verma C, Quraishi MA (2017) Experimental and quantum chemical analysis of 2-amino-3-((4-((S)-2-amino-2-carboxyethyl)-1H-imidazol-2-yl)thio) propionic acid as new and green corrosion inhibitor for mild steel in 1M hydrochloric acid solution. J Mol Liq C. https://doi.org/10.1016/j.molliq.2016.11.011
Desimone MP, Gordillo G, Simison SN (2011) The effect of temperature and concentration on the corrosion inhibition mechanism of an amphiphilic amido-amine in CO2 saturated solution. Corros Sci 53:4033–4043. https://doi.org/10.1016/j.corsci.2011.08.009
Arrousse N, Salim R, Bousraf FZ et al (2022) Experimental and theoretical study of xanthene derivatives as corrosion inhibitor for mild steel in hydrochloric acid solution. J Appl Electrochem 52:1275–1294. https://doi.org/10.1007/s10800-022-01705-x
El Hajjaji F, Abrigach F, Hamed O et al (2018) Corrosion resistance of mild steel coated with organic material containing pyrazol moiety. Coatings 8:330. https://doi.org/10.3390/coatings8100330
Arrousse N, Salim R, Abdellaoui A et al (2021) Synthesis, characterization, and evaluation of xanthene derivative as highly effective, nontoxic corrosion inhibitor for mild steel immersed in 1 M HCl solution. J Taiwan Inst Chem Eng 120:344–359. https://doi.org/10.1016/j.jtice.2021.03.026
Matin MA, Islam MM, Bredow T, Aziz MA (2017) The effects of oxidation states, spin states, and solvents on molecular structure, stability and spectroscopic properties of Fe-catechol complexes: a theoretical study. Adv Chem Eng Sci 7:137–153. https://doi.org/10.4236/aces.2017.72011
Zhan C-G, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107:4184–4195. https://doi.org/10.1021/jp0225774
Savas KNI (2018) Conceptual density functional theory and its application in the chemical domain. Apple Academic Press, New York
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Acknowledgements
The authors would like to thank the Director of Molecular Chemistry and Natural Substance, Moulay Ismail University, Faculty of Science, Meknes, and the Director of Engineering Laboratory of Organometallic, Molecular Materials, and Environment, Faculty of Sciences, University Sidi Mohamed Ben Abdellah, Fez. Morocco.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZM did the experimental part and wrote the manuscript, WE did the experimental part, DZ wrote the results for the theoretical part, Ouassima RIFFI contributed to the production of the figures and the tables, and MT and AA revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
M’hamdi, Z., Riffi, O., Ettahiri, W. et al. Investigation into the Prevention of Environmental Degradation of Mild Steel in a 1M HCl Solution Using Extracts Derived from Pelargonium graveolens. J Bio Tribo Corros 9, 82 (2023). https://doi.org/10.1007/s40735-023-00800-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-023-00800-6