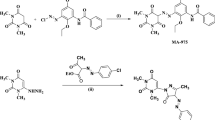
Abstract
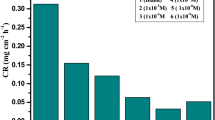
Some perimidin-10-one derivatives (1–3) were investigated for corrosion protection of copper in nitric acid solution 2.0 M using mass loss (ML), electrochemical frequency modulation (EFM), electrochemical impedance spectroscopy (EIS), and Tafel polarization techniques. At an optimum dose of 1.1 × 10–5 M, perimidin-10-one derivatives (1–3) provide 88.8% hindrance. These compounds were predominantly working as mixed inhibitors, according to Tafel. A corrosion hindrance mechanism was also devised using the EIS test. The Florry–Huggins isotherm governs the adsorption of perimidin-10-one derivatives (1–3) on the surface of Cu. The parameters of thermodynamic activation were estimated to build a corrosion hindrance mechanism. EDX and SEM were used to evaluate the morphology of protected copper. The results of experimental study were confirmed by theoretical analyses. The results of experimental study were confirmed by theoretical analyses. Quantum chemical calculations and molecular dynamic simulations have been used to apply theoretical studies. The low energy gap, mulliken, and fukui indices are all visible in quantum chemical computations. Compound 1 has a higher adsorption energy than the other compounds, according to the results of the molecular dynamics simulation. The following is the order in which the inhibition efficiency is regulated: (1) > (2) > (3).
Graphical Abstract


























Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Toumiat K, Guibadj A, Taouti MB (2017) Copper corrosion inhibition using btah inhibitor in sodium chloride medium: experimental and theoretical studies. Eurasian J Anal Chem 12(3):275–294. https://doi.org/10.12973/ejac.2017.00170a
Satpati AK, Reddy AVR (2011) Electrochemical study on corrosion inhibition of copper in hydrochloric acid medium and the rotating ring-disc voltammetry for studying the dissolution. Int J Electrochem 2011:173462–1734609. https://doi.org/10.4061/2011/173462
Rahal HT, Abdel-Gaber AM, Younes GO (2016) Inhibition of steel corrosion in nitric acid by sulfur containing compounds. Chem Eng Commun 203:435–445. https://doi.org/10.1080/00986445.2015.1017636
Karthik G, Sundaravadivelu M, Rajkumar P, Manikandan M (2015) Diaza-adamantane derivatives as corrosion inhibitor for copper in nitric acid medium. Res Chem Intermed 41:7593–7615. https://doi.org/10.1007/s11164-014-1846-8
Tansuğ G, Tüken T, Giray ES, Fındıkkıran G, Sığırcık G, Demirkol O, Erbil M (2014) A new corrosion inhibitor for copper protection. Corr Sci 84:21. https://doi.org/10.1016/j.corsci.2014.03.004
Feroz-Khan P, Shanthi V, Babu RK, Muralidharan S, ChandraBanik R (2015) Effect of benzotriazole on corrosion inhibition of copper under flow conditions. J Environ Chem Eng 3(1):10–19. https://doi.org/10.1016/j.jece.2014.11.005
Vrsalović L, Gudić S, Gracić D, Smoljko I, Ivanić I, Kliškić M, Oguzie EE (2018) Corrosion protection of copper in sodium chloride solution using Propolis. Int J Electrochem Sci 13:2102–2117. https://doi.org/10.20964/2018.02.71
Udochukwu Ofoegbu S, Galvão TLP, Gomes JRB, Tedim J, Nogueira HIS, Ferreira MGS, Zheludkevichac ML (2017) Corrosion inhibition of copper in aqueous chloride solution by 1H–1,2,3-triazole and 1,2,4-triazole and their combinations: electrochemical, Raman and theoretical studies. Phys Chem Chem Phys 19:6113–6129. https://doi.org/10.1039/c7cp00241f
Feng L, Zhang S, Qiang Y, Xu Y, Guo L, Madkour LH, Chen S (2018) Experimental and theoretical investigation of thiazolyl blue as a corrosion inhibitor for copper in neutral sodium chloride solution. Materials 11:1042–1059. https://doi.org/10.3390/ma11061042
Arshad N, Rehman Akram A, Akram M, Rasheed I (2017) Triazolothiadiazine derivatives as corrosion inhibitors for copper, mild steel and aluminum surfaces: electrochemical and quantum investigations. Prot Met Phys Chem Surf 53(2):343–358. https://doi.org/10.1134/S2070205117020046
Fouda AEAS, Etaiw SH, El-Azziz DMA, Elbaz OA (2017) Synergistic effect of barium chloride on corrosion inhibition of copper by aqueous extract of lupine seeds in nitric acid. Int J Electrochem Sci 12:5934–5950. https://doi.org/10.0964/2017.07.08
Fouda AS, Fouad RR (2016) New azonitrile derivatives as corrosion inhibitors for copper in nitric acid solution, Cogent. Chemistry 2:1221174–1221188. https://doi.org/10.1080/23312009.2016.1221174
Zarrouk, Hammouti B, Zarrok H, Bouachrine M, Khaled KF, Al-Deyab SS (2012) Corrosion inhibition of copper in nitric acid solutions using a new triazole derivative. Int J Electrochem Sci 7:89–105
Khaled KF, Amin MA (2009) Dry and wet lab studies for some benzotriazole derivatives as possible corrosion inhibitors for copper in 1.0 M HNO3. Corros Sci 51:2098–2106
Fouda AS, El-Dossoki FI, Shady IA (2018) Adsorption and corrosion inhibition behavior of polyethylene glycol on α-brass alloy in nitric acid solution. Green Chem Lett Rev 11:67–77. https://doi.org/10.1080/17518253.2018.1438525
Fiala A, Chibani A, Darchen A, Boulkamh A, Djebbar K (2007) Investigations of the inhibition of copper corrosion in nitric acid solutions by ketene dithioacetal derivatives. Appl Surf Sci 253:9347–9356. https://doi.org/10.1016/j.apsusc.2007.05.066
Howida A, Fetouh M (2014) Tarek and Abdel-Fattah, Novel plant extracts as green corrosion inhibitors for 7075–T6 aluminium alloy in an aqueous medium. Int J Electrochem Sci 9:1565–1582
Abiola OK, James AO (2010) The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci 52:661–664. https://doi.org/10.1016/j.corsci.2009.10.026
Eldesoky AM, Hassan HM, Fouda AS (2013) Studies on the corrosion inhibition of copper in nitric acid solution using some pharmaceutical compounds. Int J Electrochem Sci 8:10376–10395
Fouda AS, Wahid HA (2016) Corrosion inhibition of copper in HNO3 solution using thiophene and its derivatives. Arab J Chem 9:S91–S99. https://doi.org/10.1016/j.arabjc.2011.02.014
Arrousse N, Salima R, Kaddouri Y, Zarrouk A, Zahri D, El Hajjaji F, Touzani R, Taleb M, Jodehd S (2020) The inhibition behavior of two pyrimidine-pyrazole derivatives against corrosion in hydrochloric solution: experimental, surface analysis and in silico approach studies. Arab J Chem 13(7):5949–5965
Echihi S, Benzbiria N, Belghiti ME, El Fal M, Boudalia M, Essassi EM, Guenbour A, Bellaouchou A, Tabyaoui M, Azzi M (2021) Corrosion inhibition of copper by pyrazole pyrimidine derivative in synthetic seawater: experimental and theoretical studies. Mater Today Proc 37(3):3958–3966
Xu Y, Zhang S, Guo L, Tan B, Liao C, Zhou Y, Madkour LH (2018) Halogen-substituted pyrazolo-pyrimidine derivatives as corrosion inhibitors for copper in sulfuric acid solution. Int J Corros Scale Inhib 7(2):236–249
Xu S, Zhang S, Guo L, Feng L, Tan B (2019) Experimental and theoretical studies on the corrosion inhibition of carbon steel by two indazole derivatives in HCl medium. Materials 12:1339–1349. https://doi.org/10.3390/ma12081339
Khaled KF (2010) Corrosion control of copper in nitric acid solutions using some amino acids—a combined experimental and theoretical study. Corros Sci 52:3225–3234. https://doi.org/10.1016/j.corsci.2010.05.039
Scendo M, Uznanska J (2011) The effect of ionic liquids on the corrosion inhibition of copper in acidic chloride solutions. Int J Corros 2011:718626–718638. https://doi.org/10.1155/2011/718626
Sherif EM, Park S-M (2006) Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim Acta 51:6556–6562. https://doi.org/10.1016/j.electacta.2006.04.047
Ignat I, Varvara S, Muresan L (2015) Studia Universitatis Babes-Bolyai, Chemia, 60(LX):127
Wei N, Jiang Y, Liu Z, Ying Y, Guo X, Wu Y, Wen Y, Yang H (2018) 4-Phenylpyrimidine monolayer protection of a copper surface from salt corrosion. RSC Adv 8:7340–7349. https://doi.org/10.1039/C7RA12256J
Farahatia R, Ghaffarinejad A, Morteza Mousavi-Khoshdel S, Rezania J, Behzadi H, Shockravid A (2019) Synthesis and potential applications of some thiazoles as corrosion inhibitor of copper in 1 M HCl: experimental and theoretical studies. Prog Org Coat 132:417–428. https://doi.org/10.1016/j.porgcoat.2019.04.005
Rhattas K, Benmessaoud M, Doubi M, Hajjaji N, Srhiri A (2011) Corrosion inhibition of copper in 3% NaCl solution by derivative of aminotriazole. Mater Sci Appl 2(4):220–225. https://doi.org/10.4236/msa.2011.24028
Fouda AS, Eldesoky AM, Diab MA, Nabih A (2016) Inhibitive, adsorption studies on carbon steel corrosion in acidic solutions by new synthesized benzene sulfonamide derivatives. Int J Electrochem Sci 11:9998–10019. https://doi.org/10.20964/2016.12.47
Sherif EM, Shamy AM, Ramla MM, ElNazhawy AOH (2007) 5-(Phenyl)-4H-1,2,4-triazole-3-thiol as a corrosion inhibitor for copper in 3.5% NaCl solutions. J Mater Chem Phys 102:231–239. https://doi.org/10.1016/j.matchemphys.2006.12.009
Valek L, Martinez S (2007) Copper corrosion inhibition by Azadirachta indica leaves extract in 0.5 M sulphuric acid. Mater Lett 61:148–151. https://doi.org/10.1016/j.matlet.2006.04.024
Farghaly TA, Mahmoud HK (2013) Synthesis, tautomeric structures, and antitumor activity of new perimidines. J Arch Pharm Chem Life Sci 346:392–402. https://doi.org/10.1002/ardp.201200486
ASTM (2004) AST G 31–72, Standard recommended practice for the laboratory immersion corrosion testing of metals. American Society for Testing and Materials, Philadelphia
Zhou L, Lv Y-L, Hu Y-X, Zhao J-H, Xia X, Li X (2018) Experimental and theoretical investigations of 1,3,5-tris(4-aminophenoxy)benzene as an effective corrosion inhibitor for mild steel in 1 M HCl. J Mol Liq 249:179–187. https://doi.org/10.1016/j.molliq.2017.10.129
Abd El-Lateef HM, Abo-Riya MA, Tantawy AH (2016) Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic Gemini surfactants on carbon steel pipelines in acid pickling processes. Corros Sci 108:94–110. https://doi.org/10.1016/j.corsci.2016.03.004
Pearson RG (1988) Absolute electronegativity and hardness application to inorganic chemistry. Inorg Chem 27:734–740
Zhang Z, Huang X, Tian N, Ni F, Ruan L, Lv Y, Wu L (2016) Corrosion inhibition effect of histidine and its derivatives self-assembled films formed of 304 stainless steel. Int J Electrochem Sci 11:9175–9191. https://doi.org/10.20964/2016.11.73
Fouda AS, Elewady GY, Shalabi K, Abdel-Aziz HK (2015) Alcamines as corrosion inhibitors for reinforced steel and their effect on cement based materials and mortar performance. RSC Adv 5:36957–36968. https://doi.org/10.1039/c5ra00717h
Gong Z, Peng Sh, Huang X, Gao L (2018) Investigation the corrosion inhibition effect of itraconazole on copper in H2SO4 at different temperatures: combining experimental and theoretical studies. Materials 11:2107–2123. https://doi.org/10.3390/ma11112107
Kumar A, Trivedi M, Sharma RK, Singh G (2017) Synthetic, spectral and structural studies of a Schiff base and its anticorrosive activity on mild steel in H2SO4. New J Chem 41:8459–8468. https://doi.org/10.1039/c7nj00896a
Fragoza-Mar L, Olivares-Xometl O, Domínguez-Aguilar MA, Flores EA, Arellanes-Lozada P, Jiménez-Cruz F (2012) Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution. Corros Sci 61:171–184. https://doi.org/10.1016/j.corsci.2012.04.031
Harckerman N, Hurd RM (1962) 1st International congress on metallic corrosion, vol 166. Butterworths, London
Lgaz H, Salghi R, Jodeh S, Hammouti B (2017) Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J Mol Liq 225:271–280. https://doi.org/10.1016/j.molliq.2016.11.039
Aoun SB (2017) On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium ionic liquid: chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv 7:36688–36696. https://doi.org/10.1039/c7ra04084a
Amin A, Khaled KF, Mohsen Q, Arida A (2010) A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros Sci 52:1684–1695. https://doi.org/10.1016/j.corsci.2010.01.019
Umoren SA, Ogbobe O, Igwe IO, Ebenso EE (2008) Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci 50:1998–2006. https://doi.org/10.1016/j.corsci.2008.04.015
Keera S, Deyab M (2005) Effect of some organic surfactants on the electrochemical behaviour of carbon steel in formation water. Colloids Surf A 266:129–140. https://doi.org/10.1016/j.colsurfa.2005.05.069
Hassan AM, Abdel-Fatah TMH (2016) Aqueous extract of Salvadora persica as a novel green corrosion inhibitor for low-alloy steel in acidic media—Part I. Int J Electrochem Sci 11:6959–6975. https://doi.org/10.20964/2016.08.48
Abd El Rehim S, Hassan H, Amin M (2003) The corrosion inhibition study of sodium dodecyl benzene sulphonate to aluminium and its alloys in 1.0 M HCl solution. Mater Chem Phys 78:337–348. https://doi.org/10.1016/S0254-0584(01)00602-2
Hammouti B, Dafali A, Touzani R, Bouachrine M (2012) Inhibition of copper corrosion by bipyrazole compound in aerated 3% NaCl. Saudi Chem Soc 16:413–418. https://doi.org/10.1016/j.jscs.2011.02.009
Mu G, Li X, Liu G (2005) Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0.5 M sulfuric acid. Corros Sci 47:1932–1952. https://doi.org/10.1016/j.corsci.2004.09.020
Putilova J, Balezin S, Bacannik IN, Bishop VP (1960) Metal corrosion inhibitors. Pergamon, Oxford, p 1196
Bourazmi H, Tabyaoui M, El Hattabi L, El Aoufir Y, Ebenso EE, Ansari A (2018) Camphor as an effective corrosion inhibitor for carbon steel in 1 M HCl solution: electrochemical and quantum chemical investigation. J Mater Environ Sci 9(3):1058–1074. https://doi.org/10.26872/jmes.2017.9.3.118
El Faydy M, Touir R, Ebn Touhami M, Zarrouk A, Jama C, Lakhrissi B, Olasunkanmi LO, Ebenso EE, Bentiss F (2018) Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: experimental, DFT and Monte Carlo simulation studies. Phys Chem Chem Phys 20:20167–20187. https://doi.org/10.1039/c8cp03226b
Motawea MS, Abdelaziz MA (2015) Some pyrazole derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Eur J Chem 6(3):342–349. https://doi.org/10.5155/eurjchem.6.3.342-349.1279
Savita, Mourya P, Chaubey N, Kumar S, Singh VK, Singh MM (2016) Strychnos nuxvomica, Piper longum and Mucuna pruriens seed extracts as eco-friendly corrosion inhibitors for copper in nitric acid. RSC Adv 6:95644–95655. https://doi.org/10.1039/c6ra16481a
Zarrouk A, Hammouti B, Dafali A, Bentiss F (2013) Inhibitive properties and adsorption of purpald as a corrosion inhibitor for copper in nitric acid medium. Ind Eng Chem Res 52:2560–2568. https://doi.org/10.1021/ie301465k
Zheng X, Gong M, Li Q, Guo L (2018) Corrosion inhibition of mild steel in sulfuric acid solution by loquat (Eriobotrya japonica Lindl.) leaves extract. Sci Rep 8:9140. https://doi.org/10.1038/s41598-018-27257-9
Gadow HS, Motawea MM (2017) Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv 7:24576–24588. https://doi.org/10.1039/c6ra28636d
Oukhrib R, El Issamia, El Ibrahimi B, El Mouadena K, Bazzi L, Bammou L, Chaouay A, Salghi R, Jodeh S, Hammouti B, Amin-Alami A (2017) Ziziphus lotus as green inhibitor of copper corrosion in natural sea water. Portugaliae Electrochim Acta 35(4):187–200. https://doi.org/10.4152/pea.201704187
Talati JD, Modi RM (1986) Inhibition of corrosion of aluminium–copper alloy in NaOH. Trans SAEST 11:295
Thomas JM, Thomas WJ (1981) Introduction to the principles of heterogeneous catalysis, 5th edn. Academic Press, London, p 14
Putilova IN, Balezin SA, Barannik VP (1960) Metallic corrosion inhibitors. Pergamon Press, New York, p 31
Riggs OL Jr, Hurd RM (1967) Temperature coefficient of corrosion inhibition. Corrosion 23:252–260
Martinez S, Stern I (2001) Inhibitory mechanism of low-carbon steel corrosion by mimosa tannin in sulphuric acid solution. J Appl Electrochem 31:973–978
Abd El-Rehim SS, Ibrahim MAM, Khalid KF (2001) The inhibition of 4-(2′-amino-5′-methylphenylazo) antipyrine on corrosion of mild steel in HCl solution. Mater Chem Phys 70:268–273
Li X, Mu G (2005) Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid: weight loss study, electrochemical characterization, and AFM. Appl Surf Sci 252:1254–1265
Tang L, Mu G, Liu G (2003) The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros Sci 45:2251–2262
Li X, Tang L (2005) Synergistic inhibition between OP and NaCl on the corrosion of cold-rolled steel in phosphoric acid. Mater Chem Phys 90:286–297
Laidler KJ (1963) Reaction kinetics, vol 1, 1st edn. Pergamon Press, New York
Zhang QB, Hua YX (2009) Corrosion inhibition of mild steel by alkyl imidazolium ionic liquids in hydrochloric acid. Electrochim Acta 54:1881–1887. https://doi.org/10.1016/j.electacta.2008.10.025
Krid F, Zouaoui E, Salah M (2018) Aqueous extracts of Opuntia ficus-indica as a green corrosion inhibitor of A283C carbon steel in sulfuric acid solution. Chem. Chem. Technol. 12(3):405–409. https://doi.org/10.23939/chcht12.03.405
Kamal C, Sethuraman MG (2012) Caulerpin—a bis-Indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga Caulerpa racemose. Ind Eng Chem Res 51:10399–10407. https://doi.org/10.1021/ie3010379
Amin MA, Ahmed MA, Arida HA, Kandemirli F, Saracoglu M, Arslan T, Basaran MA (2011) Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part III. Immersion time effects and theoretical studies. Corros Sci 53:1895–1909. https://doi.org/10.1016/j.corsci.2011.02.007
Moretti G, Guidi F, Fabris F (2013) Corrosion inhibition of the mild steel in 0.5 M HCl by 2-butyl-hexahydropyrrolo[1,2-b][1,2]oxazole. Corros Sci 76:206–218. https://doi.org/10.1016/j.corsci.2013.06.044H
Hamani H, Douadi T, Daoud D, Al-Noaimi M, Rikkouh RA, Chafaa S (2017) 1-(4-Nitrophenyl-imino)-1-(phenylhydrazone)-propane-2-one as a corrosion inhibitor for mild steel in 1 M HCl solution: Weight loss, electrochemical, thermodynamic and quantum chemical studies. J Electroanal Chem 801:425–438. https://doi.org/10.1016/j.jelechem.2017.08.031
Laamari MR, Benzakour J, Berrekhis F, Abouelfida A, Derja A, Villemin D (2016) Adsorption and corrosion inhibition of carbon steel in hydrochloric acid medium by hexamethylenediamine tetra (methylene phosphonic acid). Arab J Chem 9:S245–S251. https://doi.org/10.1016/j.arabjc.2011.03.018
Eldesoky AM, Diab MA, El-Sonbati AZ, Salam SF (2017) Anti-corrosive properties of new eco-friendly dimethylamino compounds on C-steel corrosion in 2 M HCl. Int J Electrochem Sci 12:4215–4237. https://doi.org/10.20964/2017.05.73
Sing HA, Ebenso EE, Quraishi MA (2012) Stem extract of brahmi (Bacopa monnieri) as green corrosion inhibitor for aluminum in NaOH solution. Int J Electrochem Sci 7:3409–3419
Bothi Raja P, Sethuraman MG (2008) Atropine sulphate as corrosion inhibitor for mild steel in sulphuric acid medium. Mater Lett 62:1602–1604. https://doi.org/10.1016/j.matlet.2007.09.032
Pourbaix M (1975) Atlas of electrochemical equilibria in aqueous solutions. NACE, Houston
Johnson HE, Leja J (1965) On the potential/pH diagrams of the Cu–NH–H2O and Zn–NH–H2O systems. J Electrochem Soc 112:638–641. https://doi.org/10.1149/1.2423629
Fouda AS, Badawya AA (2019) Adsorption and corrosion inhibition of Cu in nitric acid by expired simvastatin drug. Prot Met Phys Chem Surf 55:572–582. https://doi.org/10.1134/S2070205119030146
Yan Y, Li W, Cai L, Hou B (2008) Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim Acta 53:5953–5960. https://doi.org/10.1016/j.electacta.2008.03.065
Chen J, Qiang Y, Peng S, Gong Z, Zhang S, Gao L, Tan B, Chen S, Guo L (2018) Experimental and computational investigations of 2-amino-6-bromobenzothiazole as a corrosion inhibitor for copper in sulfuric acid. J Adhes Sci Technol 32(19):2083–2098. https://doi.org/10.1080/01694243.2018.1460948
Macdonald DD, Mckubre MCH (1982) Impedance measurements in electrochemical systems. In: Bockris JOM, Conway BE, White RE (eds) Modern aspects of electrochemistry, vol 14. Plenum Press, New York, p 61
Ali SM, Al Lehaibi HA (2016) Control of zinc corrosion in acidic media: green fenugreek inhibitor. Trans Nonferrous Met Soc China 26:3034–3045. https://doi.org/10.1016/S10036326(16)64434-5
Galai M, Benqlilou H, Ebn Touhami M, Belhaj T, Berrami K, ElKafssaoui H (2018) Comparative analysis for the corrosion susceptibility of copper alloys in sandy soil. Environ. Eng. Res. 23(2):164–174. https://doi.org/10.4491/eer.2017.077
Outirite M, Lagrenee M, Lebrini M, Traisnel M, Jama C, Vezin H, Bentiss F (2010) Ac impedance, X-ray photoelectron spectroscopy and density functional theory studies of 3,5-bis(n-pyridyl)-1,2,4-oxadiazoles as efficient corrosion inhibitors for carbon steel surface in hydrochloric acid solution. Electrochim Acta 55:1670–1681. https://doi.org/10.1016/j.electacta.2009.10.048
Muthukrishnan P, Prakash P, Jeyaprabha B, Shankar K (2015) Stigmasterol extracted from Ficus hispida leaves as a green inhibitor for the mild steel corrosion in 1 M HCl solution. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.09.005
Mo S, Qin TT, Luo HQ, Li NB (2015) Insights into the corrosion inhibition of copper in hydrochloric acid solution by self-assembled films of 4-octylphenol. RSC Adv 5:90542–90549. https://doi.org/10.1039/C5RA13074C
Chen W, Hong S, Xiang B, Luo H, Li M, Li N (2013) Corrosion inhibition of copper in hydrochloric acid by coverage with trithiocyanuric acid self-assembled, monolayers. Corros Eng Sci Technol 48(2):98–107. https://doi.org/10.1179/1743278212Y.0000000053
Jwad Habeeb H, Mohammed Luaibi H, Mohammed Dakhil R, Abdul Amir Kadhum H, Ahmed Al-Amiery A, Sumer Gaaz T (2018) Development of new corrosion inhibitor tested on mild steel supported by the electrochemical study. Results Phys 8:1260–1267. https://doi.org/10.1016/j.rinp.2018.02.015
Preethi Kumari P, Shetty P, Rao SA (2017) Electrochemical measurements for the corrosion inhibition of mild steel in 1 M hydrochloric acid by using an aromatic hydrazide derivative. Arab J Chem 10:653–663. https://doi.org/10.1016/j.arabjc.2014.09.005
Kong P, Feng H, Chen N, Lu Y, Li S, Wang P (2019) Polyaniline/chitosan as a corrosion inhibitor for mild steel in acidic medium. RSC Adv 9:9211–9217. https://doi.org/10.1039/c9ra00029a
Volpi E, Foiadelli C, Trasatti S, Koleva D (2017) Development of smart corrosion inhibitors for reinforced concrete structures exposed to a microbial environment. Ind Eng Chem Res 56(20):5778–5794. https://doi.org/10.1021/acs.iecr.7b00127
Ali IH, Suleiman MHA (2018) Effect of acid extract of leaves of Juniperus procera on corrosion inhibition of carbon steel in HCl solutions. Int J Electrochem Sci 13:3910–3922. https://doi.org/10.20964/2018.04.01
Idusuyi N, Ajide OO, Oluwole OO, Arotiba OA (2017) Electrochemical impedance study of an Al6063–12%SiC–Cr composite immersed in 3 wt.% sodium chloride. Procedia Manuf 7:413–419
Mohan R, Joseph A (2018) Corrosion protection of mild steel in hydrochloric acid up to 313 K using propyl benzimidazole electroanalytical, adsorption and quantum chemical studies. Egypt J Pet 27(1):11–20. https://doi.org/10.1016/j.ejpe.2016.12.003
Benabdellah M, Tounsi A, Khaled KF, Hammouti B (2011) Thermodynamic, chemical and electrochemical investigations of 2-mercapto benzimidazole as a corrosion inhibitor for mild steel in hydrochloric acid solutions. Arab J Chem 4:17–24. https://doi.org/10.1016/j.arabjc.2010.06.010
Guo W, Chen S, Serb HMA (2006) A study of the inhibition of copper corrosion by triethyl phosphate and triphenyl phosphate self-assembled monolayers. Serb Chem Soc 71(2):167–175. https://doi.org/10.2298/JSC0602167G
Amin MA, Mersal GAM, Mohsen Q (2011) Monitoring corrosion and corrosion control of low alloy ASTM A213 grade T22 boiler steel in HCl solutions. Arab J Chem 4(2):223–229. https://doi.org/10.1016/j.arabjc.2010.06.040
Shalabi K, Abdallah YM, Fouda AS (2015) Corrosion inhibition of aluminum in 0.5 M HCl solutions containing phenyl sulfonylacetophenoneazo derivatives. Res Chem Intermed 41:4687–4711. https://doi.org/10.1007/s11164-014-1561-5
Fouda AS, Nazeer AA, Saber A (2014) Electrochemical adsorption properties and inhibition of zinc corrosion by two chromones in sulfuric acid solutions. J Korean Chem Soc 58:160–168. https://doi.org/10.5012/jkcs.2014.58.2
El-Haddad MN (2016) Inhibitive action and adsorption behavior of cefotaxime drug at copper/hydrochloric acid interface: electrochemical, surface and quantum chemical studies. RSC Adv 6:57844–57853. https://doi.org/10.1039/c6ra03316d
El-Haddad MN, Fouda AS (2013) Corrosion inhibition and adsorption behavior of some azo dye derivatives on carbon steel in acidic medium : synergistic effect of halide ions. Chem Eng Commun 200:1366–1393. https://doi.org/10.1080/00986445.2012.746675
Bosch RW, Bogaerts WF, Syrett B (2003) Proceedings of 8th international symposium on electrochemical methods in corrosion research modulation (EFM) technique, Nieuwpoort, Belgium, 4–9 May 2003
Motawea MM, El-Hossiany A, Fouda AS (2019) Corrosion control of copper in nitric acid solution using Chenopodium extract. Int J Electrochem Sci 14:1372–1387. https://doi.org/10.20964/2019.02.29
Mara Cortez Alves de Oliveira V, Aguiara C, Muci Vazqueza A, Laurent Marie Robin A, Justino Ribeiro Barboza M (2017) Corrosion behavior analysis of plasma-assisted PVD coated Ti–6Al–4V alloy in 2 M NaOH solution. Mater Res 20:436–404
Prabhu R, Venkatesha T, Shanbhag A, Kulkarni G, Kalkhambkar R (2008) Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros Sci 50:3356–3362. https://doi.org/10.1016/j.corsci.2008.09.009
Idouhli R, N’Ait Outside A, Koumya Y, Abouelfida A, Benyaich A, Auhmani A, Itto MYA (2018) Electrochemical studies of monoterpenic thiosemicarbazones as corrosion inhibitor for steel in 1 M HCl. Int J Corros 2018:9212705–9212719. https://doi.org/10.1155/2018/9212705
Lopez DA, Simison SN, de Sanchez SR (2005) Inhibitors performance in CO2 corrosion EIS studies on the interaction between their molecular structure and steel microstructure. Corros Sci 47:735–755. https://doi.org/10.1016/j.corsci.2004.07.010
Satapathy AK, Gunasekaran G, Sahoo Kumar SC, Rodrigues APV (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856. https://doi.org/10.1016/j.corsci.2009.08.016
Ebenso EE, Arslan T, Kandemirli F, Caner N, Love I (2010) Quantum chemical studies of some rhodanine azosulpha drugs as corrosion inhibitors for mild steel in acidic medium. Int J Quant Chem 110:1003–1018. https://doi.org/10.1002/qua.22249
Ozcan M, Dehri I, Erbil M (2004) Organic sulfur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl Surf Sci 236:155–164. https://doi.org/10.1016/j.apsusc.2004.04.017
Ansari KR, Ramkumar S, Nalini D, Quraishi MA (2016) Studies on adsorption and corrosion inhibitive properties of quinoline derivatives on N80 steel in 15% hydrochloric acid. Cogent Chem 2:1145032–1145045. https://doi.org/10.1080/23312009.2016.1145032
Mert BD, Mert ME, Kardas ME, Yazici G (2011) Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros Sci 53:4265–4272. https://doi.org/10.1016/j.corsci.2011.08.038
Roque JM, Pandiyan T, Cruz J, Garcl’a-Ochoa E (2008) DFT and electrochemical studies of tris(benzimidazole-2ylmethyl)amine as an effective corrosion inhibitor for carbon steel surface. Corros Sci 50:614–624. https://doi.org/10.1016/j.corsci.2007.11.012
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981. https://doi.org/10.1016/j.corsci.2008.08.043
Musa AY, Kadhum AH, Mohamad AB, Takriff MS (2010) Experimental and theoretical study on the inhibition performance of triazole compounds for mild steel corrosion. Corros Sci 52:3331. https://doi.org/10.1016/j.corsci.2010.06.002
Lukovits I, Lalman E, Zucchi F (2001) Corrosion inhibitors—correlation between electronic structure and efficiency. Corrosion 57:3–8. https://doi.org/10.5006/1.3290328
Zhang W, Liu Y, Zhang Y, Wang L-J, Wu Y-C, Li H-J (2020) 9-Substituted acridines as effective corrosion inhibitors for mild steel: electrochemical, surface morphology, and computational studies. New J Chem 44:6464–6474. https://doi.org/10.1039/d0nj00440e
Boughoues Y, Benamir M, Messaadi L, Bouider N, Abdelaziz S (2020) Experimental and theoretical investigations of four amine derivatives as effective corrosion inhibitors for mild steel in HCl medium. RSC Adv 10:24145–24158. https://doi.org/10.1039/d0ra03560b
Qiang Y, Zhang S, Tan B, Chen S (2018) Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros Sci 133:6–16. https://doi.org/10.1016/j.corsci.2018.01.008
Shalabi K, Abdallah YM, Hassan HM, Fouda AS (2014) Adsorption and corrosion inhibition of Atropa belladonna extract on carbon steel in 1 M HCl solution. Int J Electrochem Sci 9:1468–1487
Cerny V (1985) Thermodynamical approach to the traveling salesman problem: an efficient simulation algorithm. J Optim Theor Appl 45:41–55
Dagdag O, Safi Z, Erramli H, Cherkaoui O, Wazzan N, Guo L, Verma C, Ebenso E, El Harfia A (2019) Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: computational studies and sustained experimental studies. RSC Adv 9:14782–14796. https://doi.org/10.1039/c9ra01672d
Fouda AS, Shalabi K, Idress AA (2015) Ceratonia siliqua extract as a green corrosion inhibitor for copper and brass in nitric acid solutions. Green Chem Lett Rev 8:17–29. https://doi.org/10.1080/17518253.2015.1073797
Qiang Y, Zhang S, Xu S, Li W (2016) Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interfaces Sci 472:52–59. https://doi.org/10.1016/j.jcis.2016.03.023
Qiang Y, Zhang S, Guo L, Xu S, Feng L, Obot IB, Chen S (2017) Sodium dodecyl benzene sulfonate as a sustainable inhibitor for zinc corrosion in 26% NH4Cl solution. J Clean Prod 152:17–25. https://doi.org/10.1016/j.jclepro.2017.03.104
Wang J, Qiang Y, Jiang L, Xiang B, Chen S, Xing S, Wang Y, Wang Y (2018) Excellent inhibition performance of low-toxicity dibenzyldithiocarbamic acid zinc salt selfassemble nano-film for copper corrosion in sulfuric acid. J Mol Liq 271:959–969. https://doi.org/10.1016/j.molliq.2018.09.061
Putilova IN, Balezin SA, Barannik VP (1960) Corrosion inhibitors. Pergamum Press, Oxford, p 85
Evans UR (1990) The corrosion and oxidation of metals. Edward Arnold, London, pp 324, 326
Shams El Din AM, Fakhr MY (1974) A thermometric study of the reaction between fe and HNO3. Corros Sci 14:635–644
Ellingham HJT (1932) J Chem Soc 15:65
Balezin SA, Parfenov GP (1953) Russ J Appl Chem 26:795
Benahmed M, Djeddi N, Akkal S, Laouer H (2016) Saccocalyx satureioides as corrosion inhibitor for carbon steel in acid solution. Int J Ind Chem 7:109–120. https://doi.org/10.1007/s40090-016-0082-z
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407. https://doi.org/10.1186/1752-153X-7-83
Baeza H, Guzman M, Ortega P, Vera L (2003) Corrosion inhibition of copper in 0.5 M hydrochloric acid by 1,2,3-thiadiazole-2,5-dithiol. J Chil Chem Soc 48:717–732. https://doi.org/10.4067/S0717-97072003000300004
Tan B, Zhang S, Liu H, Qiang Y, Li W, Guo L, Chen S (2019) Insights into the inhibition mechanism of three 5-phenyltetrazole derivatives for copper corrosion in sulfuric acid medium via experimental and DFT methods. J Taiwan Inst Chem Eng 102:424–437. https://doi.org/10.1016/j.jtice.2019.06.005
Karthik G, Sundaravadivelu M (2016) Investigations of the inhibition of copper corrosion in nitric acid solutions by levetiracetam drug. Egypt J Pet 25:481–493. https://doi.org/10.1016/j.ejpe.2015.10.009
Fouda AS, Ismael MA, AboShahba RM, Kamel LA, El-Nagggar AA (2017) Corrosion inhibition of copper and α-brass in 1 M HNO3 solution using new aryl pyrimido [5, 4-c] quinoline-2,4-dione derivative. Int J Electrochem Sci 12:3361–3384. https://doi.org/10.20964/2017.04.57
Funding
There were no research Grants for this work from any funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gadow, H.S., Farghaly, T.A. & Eldesoky, A.M. In an Acidic Environment, Perimidin-10-one Derivatives were Evaluated as Potential Copper Corrosion Inhibitors (Experimental and Theoretical Examinations). J Bio Tribo Corros 8, 51 (2022). https://doi.org/10.1007/s40735-022-00650-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00650-8