Abstract
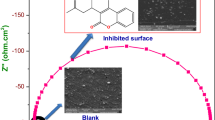
In the present study, pitting corrosion of 316 austenitic stainless steel has been investigated in 3.5% NaCl solution at temperatures of 25, 40 and 60 °C using electrochemical methods (Cyclic potentiodynamic polarization (CPP) and Electrochemical impedance spectroscopy (EIS)). Then, inhibitive effects of 2-Mercaptobenzimidazole and Polyethylenimine with different concentrations on pitting corrosion of this alloy were analyzed. Adsorption behavior of the inhibitors on the substrate surface was also investigated using Fourier-transform infrared spectroscopy (FT-IR) and atomic force microscopy (AFM). Finally, microstructural studies were used by optical microscopy (OM) and scanning electron microscopy (SEM). The results showed that pitting corrosion of this alloy was well prevented by both inhibitors. However, corrosion resistance of 2-MBI was better than corrosion resistance of PEI and the results of OM and SEM showed better adsorption of 2-MBI compared to PEI. The highest pitting potential (626 mV vs. saturated calomel electrode (SCE)) was obtained in the presence of 2-MBI, while for PEI was 410 mV vs SCE. Also, it is found that desorption of inhibitors occurred at 60 °C and the effect of good inhibition did not occur at temperatures above 60 °C (inhibition efficiency for optimum concentration of PEI at 25 and 60 °C is 90.23 and 49, respectively). EIS and cyclic polarization tests showed that thickness of the passive layer increases with increasing the inhibitor concentration, which increases alloy corrosion resistance. The result showed that 2-MBI and PEI inhibition mechanism is deposition of single protective layer and double-layer adsorption, respectively.
























Similar content being viewed by others

References
Aljourani J, Golozar MA, Raeissi K (2010) The inhibition of carbon steel corrosion in hydrochloric and sulfuric acid media using some benzimidazole derivatives. Mater Chem Phys 121:320
Markhali BP, Naderi R, Mahdavian M, Sayebani M, Arman SY (2013) Electrochemical impedance spectroscopy and electrochemical noise measurements as tools to evaluate corrosion inhibition of azole compounds on stainless steel in acidic media. Corros Sci 75:269
Sammon C, Mura C, Hajatdoost S, Yarwood J (2002) ATR-FTIR study of the diffusion and interaction of water and electrolyte solutions in polymeric membranes. J Mol Liq 96:305
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm I. Theoret Interface Sci 47:755
Finšgar M, Fassbender S, Nicolini F, Milošev I (2009) Polyethyleneimine as a corrosion inhibitor for ASTM 420 stainless steel in near-neutral saline media. Corros Sci 51:525
Ehteshamzadeh M (2006) An introduction impedance application in corrosion analysis. Sharif University of Technology Press, Tehran
El-Taib Heakal F, Hefny MM, Abd El-Tawab AM (2010) Electrochemical behavior of 304L stainless steel in high saline and sulphate solutions containing alga Dunaliella salina and β-carotene. J Alloys Compd 491:636
Jafari H, Danaee I, Eskandari H, Rashv M, Avei M (2013) Electrochemical and theoretical studies of adsorption and corrosion inhibition of N,N′-bis(2-hydroxyethoxyacetophenone)-2,2-dimethyl-1,2-propanediimine on low carbon steel (API 5L Grade B) in acidic solution. Ind Eng Chem Res 52:6617
Khatak HS, Raj B (2002) Corrosion of austenitic stainless steels, mechanism, migration and monitoring. Woodhead Publishing, Sawston
Ha HY, Lee TH, Oh CS, Kim SJ (2009) Effects of combined addition of carbon and nitrogen on pitting corrosion behavior of Fe–18Cr–10Mn alloys. Scr Mater 61:121
Khalifa MA, El-Batouti M, Mahgoub F, Bakr Aknish A (2003) Corrosion inhibition of steel in crude oil storge tanks. Mater Corros 45:251
Mehdipoura M, Naderib R, Markhali BP (2014) Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog Org Coat 77:1761
Tsutsumi Y, Nishikata A, Tsuru T (2007) Pitting corrosion mechanism of type 304 stainless steel under a droplet of chloride solution. Corros Sci 49:1394
Ahn MK, Kwon HS, Lee HM (1998) Quantitative comparison of the influences of tungsten and moleybdenum on the passivity of Fe-29Cr ferritic stainless steels. Corros Sci 40:307
Finšgar M, Fassbender S, Hirth S, Milošev I (2009) Electrochemical and XPS study of polyethyleneimines of different moleecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater Chem Phys 116:198
Migahed MA, Nassar IF (2008) Corrosion inhibition of tubing steel during acidization of oil and gas wells. Electrochim Acta 53:2877
Migahed MA, Abd-El-Raouf M, Al-Sabagh AM, Abd-El-Bary HM (2005) Effectiveness of some non ionic surfactants as corrosion inhibitors for carbon steel pipelines in oil field. Electrochim Acta 50:4683
Schmuki P, Hildebrand H, Friedrich A, Virtane S (2005) The composition of the boundary region of MnS inclusions in stainless steel and its relevance in triggering pitting corrosion. Corros Sci 47:1239
Álvarez Bustamante R, Negrón Silva G, Abreu Quijano M, Herrera Hernández H, Romero-Romo M (2009) Electrochemical study of 2-mercaptoimidazole as a novel corrosion inhibitor for steels. Electrochim Acta 54:5393
Refay SAM, Taha F, Abd El-Malak AM (2006) Corrosion and Inhibition of 316L stainless steel in natural medium by 2-mercaptobenzimidazole. Int J Electrochem Sci 1:80
Abdallah M, Salem MM, Jahdaly BA, Awad MI, Helal E, Fouda AS (2017) Corrosion inhibition of stainless steel type 316 L in 10 M HCl solution using 1,3-thiazolidin-5-one derivatives. Int J Electrochem Sci 12:4543
Olsen SR, Watanabe FS (1957) A method to determine a phosphorus adsorption maximum of soils as measured by the Langmuir isotherm. Soil Sci Soc Am J 21:144
Steszbury EE, Buchanan RA (2000) Fundamentals of electrochemical corrosion. ASM International, New York
Shet N, Nazareth R, Adimule Suchetan P (2019) Corrosion inhibition of 316 stainless steel in 2M HCl by 4-{[4-(dimethylamino)benzylidene]amino}-5-methyl-4H-1,2,4-triazole-3-thiol. Chemical Data Collections, 20
Li X, Deng S, Fum H (2011) Benzyltrimethylammonium iodide as a corrosion inhibitor for steel in phosphoric acid produced by dihydrate wet method process. Corros Sci 53:664
Jianguo Y, Lin W, Otieno Alegi V (1995) Polyvinylpyrrolidone and polyethylenimine as inhibitors for the corrosion of a low carbon steel in phosphoric acid. Corros Sci 37:975
Wei Z, Duby P (2003) Pitting inhibition of stainless steel by surfactants: an electrochemical and surface chemical approach. Colloid Interface Sci 259:97
Ahamed MR, Narren SF, Sadiq AS (2013) Synthesis of 2-mercaptobenzimidazole and some of its derivatives. J Al-Nahrain Univ 16(2):77
Edraki M, Zaarei D (2018) Modification of montmorillonite clay with 2mercaptobenzimidazole and investigation of their antimicrobial properties. Asian J Green Chem 2:189
Doneux Th, Buess-Herman Ch, Lipkowski J (2004) Electrochemical and FTIR characterization of the self-assembled monolayer of 2-mercaptobenzimidazole on Au (111). J Electroanal Chem 564:65
Lakard S, Herlem G, Lakard B, Fahys B (2004) Theoretical study of the vibrational spectra of polyethylenimine and polypropylenimine. J Mol Struct (Theochem) 685:83
Chen B-K, Su C-T, Tseng M-C, Tsay S-Y (2006) Preparation of polyetherimide nanocomposites with improved thermal. Mech Dielectr Prop Polym Bull 57:671
Yudovin-Farber I, Beyth N, Weiss EI, Domb AJ (2010) Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J Nanopart Res 12:591
Ghiamkazemi S, Amanzadeh A, Dinarvand R, Rafiee-Tehrani M, Amini M (2010) Synthesis, and characterization, and evaluation of cellular effects of the FOL-PEG-g-PEI-GAL nanoparticles as a potential non-viral vector for gene delivery. J Nanomater 863136:10
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [AF], methodology [HY], resources [ZSSR] and review and editing [YS]. The first draft of the manuscript was written by [AF] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farjami, A., Yousefnia, H., Seyedraoufi, ZS. et al. Investigation of Inhibitive Effects of 2-Mercaptobenzimidazole (2-MBI) and Polyethyleneimine (PEI) on Pitting Corrosion of Austenitic Stainless Steel. J Bio Tribo Corros 6, 96 (2020). https://doi.org/10.1007/s40735-020-00397-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00397-0