Abstract
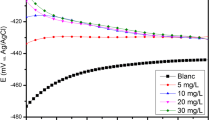
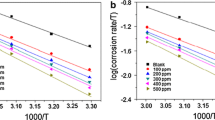
Spirulina, blue green algae is a rich source of proteins and vitamins with excellent antioxidant properties. Sunova spirulina powder an effective, green corrosion inhibitor was used to evaluate its inhibition efficiency towards mild steel in 1 M HCl medium. Weight loss studies of mild steel showed an inhibition efficiency of 96% for 600 ppm concentration of inhibitor solution and 12 h of immersion period at 303 K. The percentage of inhibition efficiency increased with a step up of 10 K raise in temperature from 303 to 333 K and thereafter decreased. The results obtained were further validated by inductively coupled plasma optical emission spectrometric (ICP-OES) measurements and electrochemical techniques that included Tafel polarisation, linear polarisation and AC impedance studies. Potentiodynamic polarisation study marked the inhibitor to be a mixed type inhibiting both cathodic and anodic reactions. The adsorption studies proved that the adsorption process was spontaneous and followed Langmuir adsorption isotherm. The thermodynamic activation and adsorption parameters calculated showed that the mechanism of inhibition involved a physisorption process initially and then it slightly shifted towards chemisorption process at higher temperature. The protective layer formed on the metal surface was studied using FTIR and SEM. The complex formation between the Fe2+ and the active constituents of the spirulina extract was verified using UV visible spectra and fluorescence spectra. The effect of inhibitor concentration and temperature on corrosion rate was tested statistically using two-way analysis of variance (ANOVA) technique.
Graphic Abstract









Similar content being viewed by others
References
Li LF, Caenen P, Celis JP (2008) Effect of hydrochloric acid on pickling of hot-rolled 304 stainless steel in iron chloride-based electrolytes. Corros Sci 50:804–810. https://doi.org/10.1016/j.corsci.2007.09.006
Ken Dibble GS, Wansbrough, H (2003) Chemical cleaning of metals. VIII-Metals-H-Cleaning-2, NZ Inst. Chem., pp 1–9
Raja PB, Ismail M, Ghoreishiamiri S et al (2016) Reviews on corrosion inhibitors: a short view. Chem Eng Commun 203:1145–1156. https://doi.org/10.1080/00986445.2016.1172485
Krishnaveni K, Ravichandran J (2014) Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Oral Oncol 50:2704–2712. https://doi.org/10.1016/S1003-6326(14)63401-4
Al-Otaibi MS, Al-Mayouf AM, Khan M et al (2014) Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J Chem 7:340–346. https://doi.org/10.1016/j.arabjc.2012.01.015
Yuli Y, Emriadi, Novesar J, Gunawarman (2015) Asian Journal of Chemistry. Asian J Chem 27:875–881
Zuo R (2007) Biofilms: Strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol 76:1245–1253. https://doi.org/10.1007/s00253-007-1130-6
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae—a review. J Algal Biomass Util 3:89–100
Wolkers H, Barbosa MJ, Kleinegris DMM, Bosma R, Wijffels RH (2011) Microalgae: the green gold of the future?: large-scale sustainable cultivation of microalgae for the production of bulk commodities. UR-Food & Biobased Research, Wageningen, p 32
Colla LM, Oliveira Reinehr C, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493. https://doi.org/10.1016/j.biortech.2005.09.030
Sajilata MG, Singhal RS, Kamat MY (2008) Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulina platensis. Food Chem 109:580–586. https://doi.org/10.1016/j.foodchem.2008.01.005
Madhyastha HK, Vatsala TM (2007) Pigment production in Spirulina fussiformis in different photophysical conditions. Biomol Eng 24:301–305. https://doi.org/10.1016/j.bioeng.2007.04.001
Machu L, Misurcova L, Ambrozova JV et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133. https://doi.org/10.3390/molecules20011118
Piñero Estrada J (2001) Antioxidant activity of different fractions of Spirulina platensis protean extract. Farm 56:497–500. https://doi.org/10.1016/S0014-827X(01)01084-9
Rajendran S, Paulraj JR (2017) Corrosion behaviour of metals in artificial saliva in presence of spirulina powder. African J Dent 5:063–070
Sribharathy V, Rajendran S (2012) Corrosion inhibition by green inhibitor : sodium metavanadate –spirulina system correspondence. Chem Sci Rev Lett 1:25–29
Kamal C, Sethuraman MG (2012) Spirulina platensis—a novel green inhibitor for acid corrosion of mild steel. Arab J Chem 5:155–161. https://doi.org/10.1016/j.arabjc.2010.08.006
Xhanari K, Finšgar M, Knez Hrnčič M et al (2017) Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv 7:27299–27330. https://doi.org/10.1039/C7RA03944A
Badawy WA, El-rabiei MM (2014) The use of beta-carotene as environmentally safe inhibitor for Cu- Al-Ni alloyscorrosion in sulf ide polluted chloride solutions. Chem Mater Res 6:107–115
Sanat Products Ltd. https://www.sanat.co.in/health-care-products/1/sunova-spirulina-capsules
McCafferty E (2005) Validation of corrosion rates measured by the Tafel extrapolation method. Corros Sci 47:3202–3215. https://doi.org/10.1016/j.corsci.2005.05.046
Palumbo G, Berent K, Proniewicz E, Banaś J (2019) Guar gum as an eco-friendly corrosion inhibitor for pure aluminium in 1-M HCl solution. Materials (Basel) 12:2620. https://doi.org/10.3390/ma12162620
Raghavendra N (2019) Use of expired naftifine drug as corrosion inhibitor for copper in hydrochloric acid. J Adv Electrochem 5:177–179
Rugmini Ammal P, Prajila M, Joseph A (2018) Effective inhibition of mild steel corrosion in hydrochloric acid using EBIMOT, a 1, 3, 4-oxadiazole derivative bearing a 2-ethylbenzimidazole moiety: electro analytical, computational and kinetic studies. Egypt J Pet 27:823–833. https://doi.org/10.1016/j.ejpe.2017.12.004
Athar M, Ali H, Quraishi MA, Quraishi MA (2016) Corrosion inhibition of carbon steel in hydrochloric acid by organic compounds containing heteroatoms. Br Corros J 0599:1–5. https://doi.org/10.1179/000705902225002376
Huong DQ, Duong T, Nam PC (2019) Eff ect of the Structure and Temperature on Corrosion Inhibition of Thiourea Derivatives in. 1 0 M HCl Solution. ACS Omega. https://doi.org/10.1021/acsomega.9b01599
Menaka R, Subhashini S (2016) Chitosan schiff base as eco-friendly inhibitor for mild steel corrosion in 1 M HCl. J Adhes Sci Technol 30:1622–1640. https://doi.org/10.1080/01694243.2016.1156382
Alhaffar MT, Umoren SA, Obot IB, Ali SA (2018) Isoxazolidine derivatives as corrosion inhibitors for low carbon steel in HCl solution: experimental, theoretical and effect of KI studies. RSC Adv 8:1764–1777. https://doi.org/10.1039/c7ra11549k
Sanni O, Popoola API, Fayomi OSI (2019) Temperature effect, activation energies and adsorption studies of waste material as stainless steel corrosion inhibitor in sulphuric acid 0.5 M. J Bio Tribo Corros 5:1–8. https://doi.org/10.1007/s40735-019-0280-2
Riggs OL Jr, Hurd RM (1967) Temperature coefficient of corrosion inhibition. Corros. NACE 23:252–258
Menaka R, Subhashini S (2017) Chitosan schiff base as effective corrosion inhibitor for mild steel in acid medium. Polym Int. https://doi.org/10.1002/pi.5245
Tang L, Mu G, Liu G (2003) The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros Sci 45:2251–2262. https://doi.org/10.1016/S0010-938X(03)00046-5
Noor AE (2007) Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves. Int J Electrochem Sci 2:996–1017
Attar T, Larabi L, Harek Y (2014) Inhibition effect of potassium iodide on the corrosion of carbon steel (XC 38) in acidic medium. Int J Adv Chem 2:139–142. https://doi.org/10.14419/ijac.v2i2.3272
Ben Aoun S (2017) On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv 7:36688–36696. https://doi.org/10.1039/c7ra04084a
Chakravarthy MP, Mohana KN (2014) Adsorption and corrosion inhibition characteristics of some nicotinamide derivatives on mild steel in hydrochloric acid solution. ISRN Corros 2014:1–13. https://doi.org/10.1155/2014/687276
Lagren M (2002) Study of the mechanism and inhibiting 4H–1, 2, 4-triazole on mild steel corrosion in acidic media. Corros Sci 44:573–588. https://doi.org/10.1016/S0010-938X(01)00075-0
Umoren SA, Eduok UM, Solomon MM, Udoh AP (2016) Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab J Chem 9:S209–S224. https://doi.org/10.1016/j.arabjc.2011.03.008
Benabdellah M, Tounsi A, Khaled KF, Hammouti B (2011) Thermodynamic, chemical and electrochemical investigations of 2-mercapto benzimidazole as corrosion inhibitor for mild steel in hydrochloric acid solutions. Arab J Chem 4:17–24. https://doi.org/10.1016/j.arabjc.2010.06.010
Sanja Martinez IS (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Yaro AS, Khadom AA, Wael RK (2013) Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng J 52:129–135. https://doi.org/10.1016/j.aej.2012.11.001
Arukalam IO, Madu IO, Ijomah NT et al (2014) Acid corrosion inhibition and adsorption behaviour of ethyl hydroxyethyl cellulose on mild steel corrosion. J Mater 2014:1–11. https://doi.org/10.1155/2014/101709
Preethi Kumari P, Shetty P, Rao SA (2017) Electrochemical measurements for the corrosion inhibition of mild steel in 1 M hydrochloric acid by using an aromatic hydrazide derivative. Arab J Chem 10:653–663. https://doi.org/10.1016/j.arabjc.2014.09.005
Li WH, He Q, Zhang ST et al (2008) Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem 38:289–295. https://doi.org/10.1007/s10800-007-9437-7
Al-Amiery AA, Kadhum AAH, Kadihum A et al (2014) Inhibition of mild steel corrosion in sulfuric acid solution by new schiff base. Materials (Basel) 7:787–804. https://doi.org/10.3390/ma7020787
Karthikaiselvi R, Subhashini S (2014) Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-o-methoxy aniline). J Assoc Arab Univ Basic Appl Sci 16:74–82. https://doi.org/10.1016/j.jaubas.2013.06.002
El Aoufir Y, Sebhaoui J, Chaouiki A et al (2018) Two novel benzodiazepines as corrosion inhibitors for carbon steel in hydrochloric acid: experimental and computational studies. J Bio Tribo Corros. https://doi.org/10.1007/s40735-018-0169-5
Singh A, Caihong Y, Yaocheng Y et al (2019) Analyses of new electrochemical techniques to study the behavior of some corrosion mitigating polymers on n80 tubing steel. ACS Omega 4:3420–3431. https://doi.org/10.1021/acsomega.8b02983
Zehra S, Mobin M, Aslam J, Parveen M (2018) Assessment of glycine derivative N-benzylidine-2((2-oxo-2-(10H-phenothiazine-10yl)ethyl)amino) acetohydrazide as inhibitor for mild steel corrosion in 1 M HCl solution: electrochemical and theoretical approach. J Adhes Sci Technol 32:317–342. https://doi.org/10.1080/01694243.2017.1354669
Popova A, Raicheva S, Sokolova E, Christov M (1996) Frequency dispersion of the interfacial impedance at mild steel corrosion in acid media in the presence of benzimidazole derivatives. Langmuir 12:2083–2089
Acid H, Lgaz H, Masroor S et al (2020) Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Metals (Basel) 10:1–14
Parveen M, Mobin M, Zehra S, Aslam R (2018) L-proline mixed with sodium benzoate as sustainable inhibitor for mild steel corrosion in 1M HCl: an experimental and theoretical approach. Sci Rep 8:1–18. https://doi.org/10.1038/s41598-018-24143-2
Xu X, Singh A, Sun Z et al (2017) Theoretical, thermodynamic and electrochemical analysis of biotin drug as an impending corrosion inhibitor for mild steel in 15% hydrochloric acid. R Soc Open Sci. https://doi.org/10.1098/rsos.170933
Singh P, Ebenso EE, Olasunkanmi LO et al (2016) Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J Phys Chem C 120:3408–3419. https://doi.org/10.1021/acs.jpcc.5b11901
Belarbi Z, Dominguez Olivo JM, Farelas F et al (2019) Decanethiol as a corrosion inhibitor for carbon steels exposed to aqueous CO2. Corrosion 75:1246–1254. https://doi.org/10.5006/3233
Alaneme KK, Olusegun SJ, Adelowo OT (2016) Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions. Alexandria Eng J 55:673–681. https://doi.org/10.1016/j.aej.2015.10.009
Shah AM, Rahim AA, Hamid SA, Yahya S (2013) PGreen inhibitors for copper corrosion by Mangrove tannin. Int J Electrochem Sci 8:2140–2153
Prabakaran M, Kim SH, Sasireka A et al (2017) β-Sitosterol isolated from rice hulls as an efficient corrosion inhibitor for mild steel in acidic environments. New J Chem 41:3900–3907. https://doi.org/10.1039/c6nj03760g
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM et al (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779. https://doi.org/10.1016/j.corsci.2005.09.017
Aloysius A, Ramanathan R, Christy A et al (2018) Experimental and theoretical studies on the corrosion inhibition of vitamins—thiamine hydrochloride or biotin in corrosion of mild steel in aqueous chloride environment. Egypt J Pet 27:371–381. https://doi.org/10.1016/j.ejpe.2017.06.003
Gadow HS, Motawea MM (2017) Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv 7:24576–24588. https://doi.org/10.1039/c6ra28636d
Dotto GL, Vieira MLG, Esquerdo VM, Pinto LAA (2013) Equilibrium and thermodynamics of azo dyes biosorption onto Spirulina platensis. Braz J Chem Eng 30:13–21. https://doi.org/10.1590/S0104-66322013000100003
Kleinegris DMM, van Es MA, Janssen M et al (2010) Carotenoid fluorescence in Dunaliella salina. J Appl Phycol 22:645–649. https://doi.org/10.1007/s10811-010-9505-y
Acknowledgements
One of the authors S.J. Hepziba wishes to thank Centre for Research, CHRIST (Deemed to be University) for providing the financial support and Ms. Jilna Jomy I MSc chemistry for her involvement in carrying out a part of the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jessima, S.J.H.M., Subhashini, S. & Arulraj, J. Sunova spirulina Powder as an Effective Environmentally Friendly Corrosion Inhibitor for Mild Steel in Acid Medium. J Bio Tribo Corros 6, 71 (2020). https://doi.org/10.1007/s40735-020-00370-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00370-x