Abstract
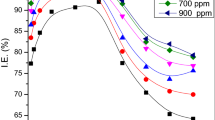
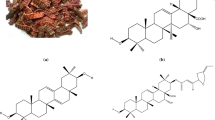
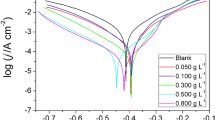
The corrosion inhibition of the Acacia catechu bark was tested on mild steel in a 0.5 M sulphuric acid solution using weight loss, Tafel and EIS. A. catechu showed its best corrosion resistance of 93.85% at a concentration of 600 mg/L. SEM and AFM were used to verify the formation of a protective layer on the surface of the mild steel. The adsorption phenomenon was verified using UV–Vis. spectroscopic technique while FT-IR confirmed the presence of different functional groups containing heteroatoms. The adsorption of the inhibitory molecules on the surface of the mild steel followed the Langmuir adsorption isotherm. Theoretical studies were carried out as a supplementary study. All the results obtained to assure that the bark extracts of A. catechu can create an effective blocking layer and control the corrosion process.









Similar content being viewed by others
References
Ahamad I, Prasad R, Quraishi MA (2010) Experimental and quantum chemical characterization of the adsorption of some Schiff base compounds of phthaloyl thiocarbohydrazide on the mild steel in acid solutions. Mater Chem Phys 124:1155–1165
Abd El-Lateef HM, Abo-Riya MA, Tantawy AH (2016) Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros Sci 108:94–110
Shahabi S, Norouzi P, Ganjali MR (2015) Electrochemical and theoretical study of the inhibition effect of two synthesized thiosemicarbazide derivatives on carbon steel corrosion in hydrochloric acid solution. RSC Adv 5:20838–20847
Bagga MK, Gadi R, Yadav OS, Kumar R, Chopra R, Singh G (2016) Investigation of phytochemical components and corrosion inhibition property of Ficus racemosa stem extract on mild steel in H2SO4 medium. J Environ Chem Eng 4:4699–4707
Hassan KH, Khadom AA, Kurshed NH (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Patel N, Rawat A, Jauhari S, Mehta G (2010) Inhibitive action on Bridelia restusa leaves extract on corrosion of mild steel in acidic media. Eur J Chem 1:129–133
Ibrahim T, Alayan H, Al Mowaqet Y (2012) The effect of Thyme leaves extract on corrosion of mild steel in HCl. Prog Org Coat 75:456–462
Al-Otaibi MS et al (2013) The effect of temperature on the corrosion inhibition of mild steel in 1 M HCL solution by Curcuma longa extract. Int J Electrochem Sci 5:847–859
Vasudha VG, Shanmuga Priya K (2013) Polyalthia longifolia as a corrosion inhibitor for mild steel in HCl solution. Res J Chem Sci 3:21–26
Garai S, Garai S, Jaisankar P, Singh JK, Elango A (2012) A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros Sci 60:193–204
Rose K, Kim BS, Rajagopal K, Arumugam S, Devarayan K (2016) Surface protection of steel in acid medium by Tabernaemontana divaricata extract: physicochemical evidence for adsorption of inhibitor. J Mol Liq 214:111–116
Oguzie EE, Chidiebere MA, Oguzie KL, Adindu CB, Momoh-Yahaya H (2014) BioLC-MS extracts for materials protection: corrosion inhibition of mild steel in acidic media by Terminalia chebula extracts. Chem Eng Commun 201:790–803
Soltani N, Tavakkoli N, Khayatkashani M, Jalali MR, Mosavizade A (2012) Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros Sci 62:122–135
Ji G, Anjum S, Sundaram S, Prakash R (2015) Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros Sci 90:107–117
Pal S, Lgaz H, Tiwari P, Chung IM, Ji G, Prakash R (2019) Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peel as green corrosion inhibitors of mild steel in aggressive chloride media. J Mol Liq 276:347–361
Tammam RH, Fekry AM, Saleh MM (2016) Understanding different inhibition actions of surfactants for mild steel corrosion in acid solution. Int J Electrochem Sci 11:1310–1326
Ameer MA, Fekry AM (2015) Corrosion inhibition by naturally occurring Hibiscus sabdariffa plant extract on mild steel alloy in HCl solution. Tur J Chem 39:1078–1088
Ahmed RA, Farghali RA, Fekry AM (2012) Study for the stability and corrosion inhibition of electrophoretic deposited chitosan on mild steel alloy in acidic medium. Int J Electrochem Sci 7:7270–7282
Fekry AM, Ameer MA (2011) Electrochemical investigation on the corrosion and hydrogen evolution rate of mild steel in sulphuric acid solution. Int J Hydrogen Energy 36:11207–11215
Ameer MA, Fekry AM (2011) Corrosion inhibition of mild steel by natural product compound. Prog Org Coat 71:343–349
Fekry AM, Gasser AA, Ameer MA (2010) Corrosion protection of mild steel by polyvinylsilsesquioxanes coatings in 3% NaCl solution. J Appl Electrochem 40:739–747
Singh K, Lal B (2006) Notes on traditional uses of khair (Acacia catechu Willd.) by inhabitants of Shivalik range in Western Himalaya. Ethnobot Leafl 10:109–112
Li XC, Yang LX, Wang HQ, Chen RY (2011) Phenolic compounds from the aqueous extract of Acacia catechu. Chinese Chem Lett 22:1331–1334
Haldhar R, Prasad D, Saxena A, Singh P (2018) Valeriana wallichi root extract as a green & sustainable corrosion inhibitor for mild steel in acidic environments: experimental and theoretical study. Mater Chem Front 2:1225–1237
Haldhar R, Prasad D, Saxena A (2018) Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4. J Environ Chem Eng 6:2290–2301
Saxena A, Prasad D, Haldhar R (2018) Investigation of corrosion inhibition effect and adsorption activities of Cuscuta reflexa extract for mild steel in 0.5 M H2SO4. Bioelechemistry. https://doi.org/10.1016/j.bioelechem.2018.07.006
Saxena A, Prasad D, Haldhar R (2018) Investigation of corrosion inhibition effect and adsorption activities of Achyranthes aspera extract for mild steel in 0.5 M H2SO4. J Fail Anal Prev. https://doi.org/10.1007/s11668-018-0491-8
Bhardwaj N, Prasad D, Haldhar R (2018) Study of the Aegle marmelos as a green corrosion inhibitor for mild steel in acidic medium: experimenta and theoretical approach. J Bio Tribo-Corros 4:1–10
Salarvand Z, Amirnasr M, Talebian M, Raeissi K, Meghdadi S (2017) Enhanced corrosion resistance of mild steel in 1M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros Sci 114:133–145
Saxena A, Prasad D, Haldhar R (2018) Use of Asparagus racemosus extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mater Sci 53:8523–8535
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415
Kumar R, Yadav OS, Singh G (2017) Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413–427
Morad MS (2000) An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros Sci 42:1307–1326
Fekry AM, Shehata M, Azab SM, Walcarius A (2020) Voltammetric detection of caffeine in pharmacological and beverages samples based on simple nano- Co (II, III) oxide modified carbon paste electrode in aqueous and micellar media. Sens Actuators B 302:127–172
Haldhar R, Prasad D, Saxena A, Kumar A (2018) Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain Chem Pharm 9:95–105
Haldhar R, Prasad D, Saxena A (2018) Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for mild steel in 0.5 M sulphuric acid: experimental and theoretical investigations. J Environ Chem Eng 6:5230–5238
Verma C, Quraishi MA, Ebenso EE, Obot IB, El Assyry A (2016) 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1M HCl: experimental and theoretical studies. J Mol Liq 219:647–660
El-TaibHeakal F, Fekry AM (2009) Experimental and theoretical study of Uracil and Adenine inhibitors in Sn-Ag alloy/nitric acid corroding system. J Electrochem Soc 155:C534–542
Haldhar R, Prasad D, Saxena A, Kaur A (2018) Corrosion resistance of mild steel in 0.5 M H2SO4 solution by plant extract of Alkana tinctoria: experimental and theoretical studies. Eur Phys J Plus 133:356
Fekry AM, Ghoneim AA, Ameer MA (2014) Electrochemical impedance spectroscopy of chitosan coated magnesium alloys in a synthetic sweat medium. Surf Coat Technol 283:126–132
Ahmed RA, Fekry AM (2013) Preparation and characterization of a nanoparticles modified chitosan sensor and its application for the determination of heavy metals from different aqueous media. Int J Electrochem Sci 8:6692–6708
El-TaibHeakal F, Fekry AM, Fatayerji MZ (2009) Electrochemical behavior of AZ91D magnesium alloy in phosphate medium—part II. Induced passivation. J Appl Electrochem 39:1633–1642
El-TaibHeakal F, Fekry AM, Fatayerji MZ (2009) Electrochemical behavior of AZ91D magnesium alloy in phosphate medium—part I. Effect of pH. J Appl Electrochem 39:583–591
Acknowledgements
We are very thankful to Prof. Gurmeet Singh, Department of Chemistry, University of Delhi, Delhi (INDIA), for providing the lab facility for electrochemical experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haldhar, R., Prasad, D. & Bhardwaj, N. Experimental and Theoretical Evaluation of Acacia catechu Extract as a Natural, Economical and Effective Corrosion Inhibitor for Mild Steel in an Acidic Environment. J Bio Tribo Corros 6, 76 (2020). https://doi.org/10.1007/s40735-020-00368-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00368-5