Abstract
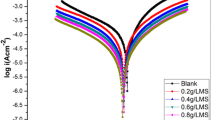
Native starch (NS) extract from sweet potato was modified by alkaline treatment. The alkaline modified starch (AMS) and NS were characterized and assessed as corrosion inhibitor of mild steel under acidic conditions by gravimetric and potentiodynamic polarization techniques. Results obtained indicate that AMS exhibited higher inhibition efficacy that was stable over time. Synergistic use of potassium iodide improved the inhibitive efficacy of AMS. Polarization data propose that AMS operated via a mixed-inhibition mechanism and the experimental adsorption data followed Langmuir isotherm. The adsorption of AMS on the metals was established by Fourier transform infrared spectroscopy and Atomic force microscopy.






Similar content being viewed by others
References
Rajendran S, Sridevi SP, Anthony N, Amairaj AJ (2005) Corrosion behavior of carbon steel in polyvinyl alcohol. Anti-Corros Methods Mater 52:102–107
Oguzie EE (2008) Corrosion inhibitive effect and adsorption behaviour of Hibiscus sabdaiffa extract on mild steel in acid media. Port Electrochem Acta 26:303–314
Rani BEA, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 38:105–120
Yurt A, Butun V, Duran B (2007) Effect of the molecular weight and structure of some novel water soluble triblock copolymers on the electrochemical behaviour of mild steel. Mater Chem Phys 105:114–121
Umoren SA, Ebenso EE (2008) Blends of polyvinyl pyrrolidone and polyacrylamide as corrosion inhibitors for aluminium in acidic medium. Ind J Chem Technol 15:355–363
Palou RM, Olivares-Xomelt O, Likhanova NV (2010) Environmentally friendly corrosion inhibitors. InTech. https://doi.org/10.5772/57252
Umoren SA, Ebenso EE, Okafor PC, Ogbobe O (2006) Water soluble polymers as corrosion inhibitors of mild steel in acidic medium. Pigment Resin Technol 35:346–352
Pawar P, Gaikwad AB, Patil PP (2007) Corrosion by protective aspects of electrochemically synthesized poly(o-anisidine-co-otoluidine) coatings on copper. Port Electrochim Acta 52:5958–5967
Torresi RM, Solange S, Pereira da Silva JE, Susana T, Torresi C (2005) Galvanic coupling between metal substrate and polyaniline acrylic blends. Corrosion protection mechanism. Electrochim Acta 50:2213–2218
de Souza S (2007) Smart coating based on polyaniline acrylic blend for corrosion protection of different metals. Surf Coat Technol 201:7574–7581
Vera R, Schrebler R, Cury P, DelRio R (2007) Corrosion protection of carbon steel and copper by polyaniline and poly(ortho-methoxyaniline) films in sodium chloride medium. Electrochemical and morphological study. J Appl Electrochem 37:519–525
Umoren SA, Obot IB (2008) Polyvinyl pyrrolidone and polyacrylamide as corrosion inhibitors for mild steel in acidic medium. Surf Rev Lett 25:277–284
Devikala S, Kamaraj P, Arthanareeswari M (2014) PVD–TiO2 composite as a corrosion inhibitor for mild steel in 3.5% NaCl. Int J Adv Chem Sci Appl 1(2):9–15
Umoren SA, Solomon MM (2014) Recent developments on the use of polymers as corrosion inhibitors—a review. Open Mater Sci J 8:39–54
Gandini A, Belgacem MN (2002) Recent contributions to the preparation of polymers derived from renewable resources. J Polym Environ 10:105–114
Ahmad S (2007) Polymer science, coatings and adhesives, safety aspects of coatings in coatings and adhesives. National Science Digital Library, NISCAIR, India
Metzger JO (2001) Organic reactions without organic solvents and oils and fats as renewable raw materials for the chemical industry. Chemosphere 43:83–87
Sugama T (1995) Pectin copolymers with organosiloxane grafts as corrosion-protective coatings for aluminium. Mater Lett 25:291–299
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62:113–116
Ren Y, Luo Y, Zhang K, Zhu G, Tan X (2008) Lignin terpolymer for corrosion inhibition of mild steel in 10% hydrochloric acid medium. Corros Sci 50:3147–3153
Solomon MM, Umoren SA, Udosoro II, Udoh AP (2010) Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros Sci 52:1317–1325
Oki M, Charles E, Alaka C, Oki TK (2011) Corrosion inhibition of mild steel in hydrochloric acid by tannins rom Rhizophora racemosa. Sci Res 2:6
Mobin M, Khan MA, Parveen M (2011) Inhibition of mild steel corrosion in acidic medium using starch and surfactants additives. J Appl Polym Sci 121:1558–1565
Sharmin E, Ahmad S, Zafar F (2012) Renewable resources in corrosion resistance. In: Shih H (ed) Corrosion resistance. InTech. www.intechopen.com/books/corrosion-resistance/renewable-resources-in-corrosion-resistance
Umoren SA, Banera MJ, Alonso-Garcia T, Gervasi CA, Mirifico MV (2013) Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 20:2529–2545
Rosliza R, Wan Nik WB (2013) Improvement of corrosion resistance of AA6061 alloy by tapioca starch in seawater. Curr Appl Phys 10(1):221–229
Rajeswari V, Kesavan D, Gopiraman M, Viswanathamurthi P (2013) Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—inhibition of cast iron corrosion. Carbohydr Polym 95:288–294
Ochoa N, Marisela B, Sancristóbal IJ, Balsamo V, Albornoz A, Joaquin L et al (2013) Modified cassava starches as potential corrosion inhibitors for sustainable development. Mater Res 16:1209–1219
Ubong ME, Mazen MK (2014) Corrosion protection of steel sheets by chitosan from shrimp shells at acid pH. Cellulose 21:3139–3143
Umoren SA, Obot IB, Madhankumar A, Gasem ZM (2015) Performance evaluation of pectin as ecofriendly corrosion inhibitor for X60 pipeline steel in acid medium: experimental and theoretical approaches. Carbohydr Polym 125:280–291
Clerici MTPS (2012) Physical and/or chemical modifications of starch by thermoplastic extrusion, thermoplastic elastomers. https://www.intechopen.com/books/thermoplastic-elastomers/physical-and-or-chemical-modifications-of-starch-by-thermoplastic-extrusion
Chen J, Jane J (1994) Preparation of granular cold-water-soluble starches by alcoholic-alkaline treatment. Cereal Chem 71:618–622
Kizil J, Seetharaman K (2002) Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J Agric Food Chem 50:3912
Mehdi JJ, Mohamadsaeed Y, Ashkan M (2014) Preparation of cold water-soluble potato starch and its characterization. J Food Sci Technol 51:601–605
Fang JM, Fowler PA, Sayers C, Williams PA (2004) The chemical modification of a range of starches under aqueous reaction condition. Carbohydr Polym 55:283–289
Han JA, Lim ST (2004) Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydr Polym 55:193–199. https://doi.org/10.1016/j.carbpol.2003.09.006
Kaur B, Fazila A, Karim AA (2011) Alcoholic-alkaline treatment of sago starch and its effect on physicochemical properties. Food Bioprod Process 89:463–471. https://doi.org/10.1016/j.fbp.2010.09.003
Rosliza R (2012) Improvement of corrosion resistance of aluminium alloy by natural products. In: Shih H (ed) Corrosion resistance. InTech, pp 377–396. ISBN 978-953-51-0467-4
Nwanonenyi SC, Madufor IC, Uzoma PC, Chukwujike IC (2016) Corrosion inhibition of Mild Steel in sulphuric acid environment using millet starch and potassium. Int Res J Pure Appl Chem 12(2):1–15
Oguzie EE (2004) Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater Chem Phys 87:212–217
Oguzie EE, Unaegbu C, Ogukwe CN, Okolue BN, Onuchukwu AI (2004) Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives. Mater Chem Phys 84:363–368
Oguzie EE, Onuoha GN, Onuchukwu AI (2005) Inhibition of aluminium corrosion in potassium hydroxide by Congo red and its synergism with halide ions. Anti-Corros Methods Mater 52:293–299
Oguzie EE, Ebenso EE (2006) Studies on the corrosion inhibiting effect of Congo red dye-halide mixtures. Pigment Resin Technol 35:30–35
Oguzie EE, Li Y, Wang FH (2007) Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J Colloid Interface Sci 310:90–98
Umoren SA, Solomon MM (2010) Effect of halide ions additives on the corrosion inhibition of aluminum in HCl by polyacrylamide. Arab J Sci Eng 35:115–129
Li X, Deng S, Lin T, Xie X, Du G (2019) Cassava starch ternary graft copolymer as a corrosion inhibitor for steel in HCl solution. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2019.12.050
Suzuki T, Nishihara H, Aramaki K (1996) The synergistic inhibition effect of octylmercaptopropionate and 8-quinolinol on the corrosion of iron in an aerated 0.5 M Na2SO4 solution. Corros Sci 38:1223–1234
Eddy NO, Ebenso EE (2010) Adsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic medium. Int J Electrochem Sci 5:731–750
Shaju KS, Thomas KJ, Vinod PR, Aby P (2012) Synergistic effect of KI on corrosion inhibition of mild steel by polynuclear Schiff base in sulphuric acid. Hindawi. https://doi.org/10.5402/2012/425878
Li XH, Deng SD, Fu H, Li YX (2017) Corrosion inhibition of cationic cassava starch graft copolymer for steel in HCl solution. Fine Chem 34:319–325
Li X, Deng S, Lin T, Xie X, Du G (2018) Cassava starch sodium allylsulfonate acryl amide graft copolymer as an effective inhibitor of aluminium corrosion in HCl solution. J Taiwan Inst Chem Eng 86:252–269
Umoren SA (2008) Inhibition of aluminium and mild steel corrosion in acidic medium using gum Arabic. Cellulose 15:751–761
Eddy NO, Odiongenyi AO (2010) Corrosion inhibition and adsorption properties of ethanol extract of Heinsia crinata on mild steel in H2SO4. Pigment Resin Technol 38:288
Braga PC, Ricci D (2004) Imaging methods in atomic force microscopy. In: Methods in molecular biology. Humana Press, Totowa, pp 13–23
Yu JJ, Magonov SN (2007) Application of atomic force microscopy (AFM) in polymeric materials. Application note. Agilent Technologies, Inc., Santa Clara
Bello M, Ochoa N, Balsamo V, López-Carrasquero F, Coll S, Monsalve A et al (2010) Modified cassava starches as green corrosion inhibitors of carbon steel: an electrochemical and morphological approach. Carbohydr Polym 82:561–568. https://doi.org/10.1016/j.carbpol.2010.05.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there were no conflict of interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anyiam, C.K., Ogbobe, O., Oguzie, E.E. et al. Synergistic Study of Modified Sweet Potato Starch and KI for Corrosion Protection of Mild Steel in Acidic Media. J Bio Tribo Corros 6, 70 (2020). https://doi.org/10.1007/s40735-020-00362-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00362-x