Abstract
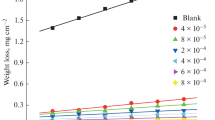
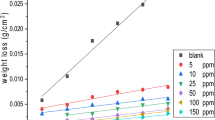
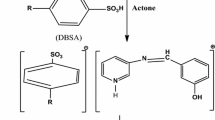
The effect of some new nonionic surfactants namely, N1,N2,N3-tris ((14-amino-3,6,9,12-tetraazatetradecyl) carbamothioyl) propane-1,2,3-tricarboxamide (compound I) and N1,N2,N3-tris((2-hydroxyethyl) carbamothioyl) propane-1,2,3-tricarboxamide (compound II) for the corrosion of low carbon steel (LCS) in 1.0 M HCl was evaluated by electrochemical and non-electrochemical techniques. From weight loss measurements, the concentration of these compounds and the temperature of the medium have a large effect on the inhibition efficiency (IE%). The IE% reached 93% for compound (I) and 77.7% for compound (II) at 75 ppm. The behavior of these compounds obeys the Temkin isotherm with good fitting. Polarization curves of these surfactants indicate that they affect both anodic metal dissolution and cathodic H2 evolution (i.e. mixed type). The action of the addition of these compounds on the LCS was detected using scanning electron microscopy, energy disperse X-ray, Fourier-transform infra-red (FTIR) spectroscopy and atomic force microscopy (AFM) techniques. Electrochemical and non-electrochemical methods gave matched results.

















Similar content being viewed by others
References
Mehdaou R, Khelifa A, Khadraoui A, Aaboubi O, HadjZiane A, Bentiss F, Zarrouk A (2016) Corrosion inhibition of carbon steel in hydrochloric acid solution by some synthesized surfactants from petroleum fractions. Res Chem Intermed 42:5509–5526
Aiad I, El-Sukkary MM, Soliman EA, El-Awady MY (2014) Inhibition of mild steel corrosion in acidic medium by some cationic surfactants. J Ind Eng Chem 20:3524–3535
Eddy NO, Momoh-Yahaya H, Oguzie EE (2015) Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1 M HCl. J Adv Res 6(2):203–217
Shaban SM, El-Sukkary MM, Soliman EA, El-Awady MY (2015) Inhibition of mild steel corrosion in acidic medium by vanillin cationic surfactants. J Mol Liq 203:20–28
Migahed MA, Zaki EG, Shaban MM (2016) Corrosion control in the tubing steel of oil wells during matrix acidizing operations. R S C Advanced 6:71384–71396
Shaban SM, Fouda AS, Rashwan SM, Ibrahim HE, El-Bhrawy MF (2016) Synthesis and characterization of newly cationic surfactants based on dimethyl amino ethoxy ethanol: physiochemical, thermodynamic and evaluation as biocide. J Mol Liq 221:224 – 234
Fouda AS, Nazeer A, El behairy WT (2018) Assessment of begonia extract as new ecofriendly inhibitor for lowcarbonsteel corrosion in acidic environment. J Bio Tribo Corrs 4(7):2–13
Singh AK, Quraishi M (2011) Investigation of the effect of disulfiram on corrosion of mild steel in hydrochloric acid solution. Corros Sci 53(4):1288–1297
Thomas S, Birbilis N, Venkatraman MS Cole IS (2012) Corrosion of zinc as a function of PH. Corrosion 68 (1):015009-015001–015009-9
Fouda AS, Ibrahim AA, El-behairy WT (2014) Thiophene derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Der Pharm Chem 6(5):144 – 157
Shaban SM, Aiad I, El-Sukkary MM, Soliman EA, El-Awady Y (2015) Evaluation of some cationic surfactants based on dimethylaminopropylamine as corrosion inhibitors. J Ind Eng Chem 21:1029–1038
Bentiss F, Lagrenee M, Traisnel M, Hornez JC (1999) The corrosion inhibition of mild steel in acidic media by a new triazolederivative. Corros Sci 41(4):789–803
Ahamad I, Quraishi MA (2009) Bis (benzimidazol-2-yl) disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros Sci 51(9):2006–2013
Abdel-Rehim SS, Khaled KF, Abd-Elshafi NS (2006) Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thioureaderivative. ElectrochimiActa 51(16): 3269–3277
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Solmaz R (2014) Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene) rhodamine. Corros Sci 79:169–176
Singh DDN, Chaudhary RS, Prakash B, Agarwal CV (1979) Inhibitive efficiency of some substituted thioureas for the corrosion of aluminium in nitric acid. Br Corros J 14(4):235–239
Shukla K, Quraishi MA (2009) 4-Substituted anilinomethylpropionate: new and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 51(9):1990–1997
Fouda AS, Al-Sarawy AA, El-Katori EE (2006) Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution. Desalination 201(1):1–13
Masroor Sh, Mobin M (2014) Non-ionic surfactant as corrosion inhibitor for aluminium in 1.0 MHCl and synergistic influence of geminisurfactant. Chem Sci Rev Lett 3(11):33–48
Fouda AS, El-desoky AM, Nabih A (2013) Inhibitive, adsorption, synergistic studies on copper corrosion in nitric acid solutions by some organic derivatives. Adv Mater Corros 2:1–15
Lakshmi SG, Tamilselvi S, Rajendran N, Babi MAK, Arivuoli D (2004) Electrochemical behaviour and characterisation of plasma nitrided Ti–5Al–2Nb–1Ta orthopaedic alloy in hanks solution. Surf Coating Technol 184(2):287–293
Mu GN, Li XH, Qu Q, Zhou J (2008) Inhibition effect of nonionic surfactant on the corrosion of cold rolled steel in hydrochloric acid. Corros Sci 50(2):420–430
Fouda AS, Mekkia D, Badr AH (2013) Extract of Camellia sinensis as green inhibitor for the corrosion of mild steel in aqueous solution. J Korean Chem Soc 57(2):264–271
Abdallah M (2002) Rhodanineazosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros Sci 44(4):717–728
Ahamad I, Prasad R, Quraishi MA (2010) Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros Sci 52(3): 933–942
El Achouri M, Gouttaya Kertit S HM, Nciri B, Bensouda Y, Pere L, Infante MR, Elkacemi K (2001) Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α,ω-bis-(dimethyl tetradecyl ammonium bromide). Prog Org Coat 43(4):267–273
Hanza AP, Naderi R, Kowsari E, Sayebani M (2016) Corrosion behavior of mild steel in H2SO4 solution with 1,4-di [1′-methylene-3′-methyl imidazolium bromide]-benzene as an ionic liquid. Corros Sci 107:96–106
Mertens SF, Xhoffer C, Decooman BC, Temmerman E (1997) Short-term corrosion of polymer-coated 55% Al-zn—part 1: behavior of thin polymer films. Corrosion 53(5):381–388
Noor EA, Al-Moubaraki AH (2008) Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styrylpyridinium iodides/hydrochloric acid systems. Mater Chem Phys 110(1):145–154
Prabhu RA, Venkatesha TV, Shanbhag AV, Kulkarni GM, Kalkhambkar RG (2008) Inhibition effects of some schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros Sci 50(12):3356–3362
Tang Y, Yang X, Yang W, Chen Y, Wan R (2010) Experimental and molecular dynamics studies on corrosion inhibition of mild steel by 2-amino-5-phenyl-1,3,4-thiadiazole. Corros Sci 52:242–249.
Caigman GA, Metcalf SK (2000) Thiophene substituted dihydropyridines. J Chem Crystallogr 30(6):415–422
Zhang J, Gong XL, Du M (2011) The inhibition mechanism of imidazoline phosphate inhibitor for Q235 steel in hydrochloric acid medium. Corros Sci 53(10):3324–3330
Awad MK, Metwally MS, Soliman SA, El-Zomrawy AA, Bedair MA (2014) Experimental and quantum chemical studies of the effect of poly ethylene glycol as corrosion inhibitors of aluminum surface. J Ind Eng Chem 20(3):796–808
Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Effect of some pyrimidinicshciff bases on the corrosion of mild steel in hydrochloric acid solution. Electrochim Acta 50(16):3446–3452
Shaban SM (2016) Studying the effect of newly synthesized cationic surfactant on silver nanoparticles formation and their biological activity. J Mol Liq 216:137–145
Negm NA, Morsy SMI (2005) Corrosion inhibition of triethanolammonium bromide mono-dibenzoate as cationic inhibitors in an acidic medium. J Surfactants Deterg 8(3):283–287
Sastri VS, Perumareddi JR (1997) Molecular orbital theoretical studies of some organic corrosion inhibitors. Corros Sci 53(8):617–622
Abdallaha M, Eltass HM, Hegazy MA, Ahmed H (2016) Adsorption and inhibition effect of novel cationic surfactant for pipelines carbon steel in acidic solution. Prot Met Phys Chem 52(4):721–730
Kellou-Kerkouche F, Benchettara A, Amara SE (2013) Anionic surfactant as a corrosion inhibitor for synthesized ferrous alloy in acidic solution. J Mater 2013:1–11
Fouda AS, Mohamed FSh, El-Sherbeni MW (2016) Corrosioninhibition of aluminum–silicon alloy in hydrochloricacid solutions using carbamidicthioanhydridederivatives. J Bio Tribo Corros 2(11):1–16
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouda, A.S., Zaki, E.G. & Khalifa, M.M.A. Some New Nonionic Surfactants Based on Propane Tricarboxylic Acid as Corrosion Inhibitors for Low Carbon Steel in Hydrochloric Acid Solutions. J Bio Tribo Corros 5, 31 (2019). https://doi.org/10.1007/s40735-019-0223-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0223-y