Abstract
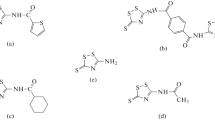
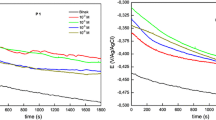
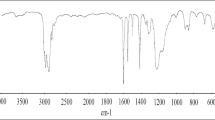
The electrochemical behavior of mild steel in phosphoric acidic solutions was investigated in the absence and presence of three heterocyclic tetrazoles derivatives using polarization and electrochemical impedance techniques. The corrosion rate is affected by both acid and inhibitors concentration. Inhibitors reduce the corrosion rate values by suppression of anodic dissolution of steel rather than the cathodic process of hydrogen evolution. Corrosion potential values were shifted to positive direction. Addition of inhibitors did not change the mechanism of corrosion process, which indicates that these compounds act by forming a film on metal surface. Impedance measurements showed that the charge transfer resistance increases and the double layer capacity decreases with addition of inhibitors. Charge transfer resistance increased with time. Maximum inhibitor efficiency was 71.5%, which leads to conclude that the studied inhibitors represent moderate anticorrosion materials. Quantum chemical investigations were also used to optimize the chemical structure of inhibitors.












Similar content being viewed by others
References
Khadom AA, Yaro AS (2011) Protection of low carbon steel in phosphoric acid by potassium iodide. Prot Met Phys Chem Surf J 47:662–669
El Bakri Y, El Aoufir Y, Bourazmi H, Harmaoui A, Sebhaoui J, Tabyaoui M, Guenbour A, Oudda H, Lgaz H, El Hajjaji F, Ben Ali A, Ramli Y, Essassi EM (2017) Corrosion control of carbon steel in phosphoric acid by 6-methyl-7H-1,2,4- triazolo[4,3-b][1,2,4]-triazepine-8(9H)-thione: Electrochemical studies. J Mater Environ Sci 8(8):2657–2666
Hegazy MA, Aiad I (2015) 1-Dodecyl-4-(((3-morpholinopropyl) imino) methyl) pyridin-1-ium bromide as a novel corrosion inhibitor for carbon steel during phosphoric acid production. J Ind Eng Chem 31:91
Khadom A (2011) Molecular structure of phenylthiourea as a corrosion inhibitor of mild steel in hydrochloric acid. Corros Sci Prot Technol 32(6):457–462
Khadom A, Musa AY, Kadhum AAH, Mohamad AB, Takriff S (2010) Adsorption kinetics of 4-amino-5-phenyl-4H-1, 2, 4-Triazole-3-thiol on mild steel surface inhibitor. Portugaliae Electrochimica Acta 28:221–230
Khadom A, Yaro AS, Kadhum AAH (2010) ‘Adsorption mechanism of benzotriazole for corrosion inhibition of coppernickel alloy in hydrochloric acid’. J Chil Chem Soc 55:150–152
Wang L (2001) Evaluation of 2-mercaptobenzimidazole as corrosion inhibitor for mild steel in phosphoric acid. Corros Sci 43:2281–2289
Noor EA (2005) The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros Sci 47:33–55
Li XH, Deng SD, Fu H (2011) Benzyltrimethylammonium iodide as a corrosion inhibitor for steel in phosphoric acid produced by dihydrate wet method process. Corros Sci 53:664–670
Poornima T, Nayak J, Shetty AN (2011) Effect of 4-(N, N-diethylamino) benzaldehyde thiosemicarbazone on the corrosion of aged 18 Ni 250 grade maraging steel in phosphoric acid solution. Corros Sci 53:3688–3696
Yaro AS, Khadom AA, Ibraheem HF (2011) Peach juice as an anti-corrosion inhibitor of mild steel. Anti-Corros Methods Mater 58(3):116–124
Benabdellah JM, Touzani R, Dafali A, Hammouti B, El Kadiri S (2007) Ruthenium–ligand complex, an efficient inhibitor of steel corrosion in H3PO4 media. Mater Lett 61:1197–1204
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Popova A, Christov M, Zwetanova A (2007) Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros Sci 49:2131–2143
Ehsani A, Bodagahi S, Shiri H, Mostaanzadeh H, Hadi M (2016) Electrochemical investigation of inhibitory of new synthesized tetrazole derivitives on corrosion of stainless steel 316L in acidic medium. Iran J Mater Sci Eng 13:19–28
Verma C, Quraishi MA, Singh A (2016) 5-Substituted 1H-tetrazoles as effective corrosion inhibitors for mildsteel in 1 M hydrochloric acid. J Taibah Univ Sci 10:718–733
Zucchi F, Trabanelli G, Fonsati M (1996) Tetrazole derivatives as corrosion inhibitors for copper in chloride solutions. Corros Sci 38:2019–2029
Dhayabaran VV, Lydia IS, Merlin JP, Srirenganayaki P (2004) Inhibition of corrosion of commercial mild steel in presence of tetrazole derivatives in acid medium. Ionics 10:123–125
Gunasekaran G, Chauhan LR (2004) Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim Acta 49:4387–4395
Zarrok H, Zarrouk A, Hammouti B, Salghi R, Jama C, Bentiss F (2012) Corrosion control of carbon steel in phosphoric acid by purpald—weight loss, electrochemical and XPS studies. Corros Sci 64:243–252
Qiang Y, Guo L, Zhang S, Li W, Yu S, Tan J (2016) Synergistic effect of tartaric acid with 2,6-diaminopyridine on the corrosion inhibition of mild steel in 0.5M HCl. Sci Rep 6:33305
Tao Z, He W, Wang S, Zhou G (2013) Electrochemical study of cyproconazole as a novel corrosion inhibitor for copper in acidic solution. Ind Eng Chem Res 52:17891–17899
Obot IB, Madhankumar A (2015) Enhanced corrosion inhibition effect of tannic acid in the presence of gallic acid at mild steel/HCl acid solution interface. J Ind Eng Chem 25:105–111
Hu K, Zhuang J, Ding J, Ma Z, Wang F, Zeng X (2017) Influence of biomacromolecule DNA corrosion inhibitor on carbon steel. Corros Sci 125:68–76
Qiang Y, Zhang S, Xu S, Li W (2016) Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J Colloid Interf Sci 472:52–59
Issa RM, Awad MK, Atlam FM (2010) DFT theoretical studies of antipyrine Schiff bases as corrosion inhibitors. Mater Corros 61:709–714
Stewart JJ (1989) Optimization of parameters for semiempirical methods II applications. J Comput Chem 10:221–264
Ozcan M, Dehri I, Erbil M (2004) Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl Surf Sci 236:155–164
Khadom AA, Yaro AS, Musa AY, Mohamad AB, Kadhum AAH (2012) Corrosion inhibition of copper-nickel alloy: experimental and theoretical studies. J Korean Chem Soc 56(4):406–415
Musa AY, Kadhum AH, Mohamad AB, Takriff MS (2010) On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1, 2, 4-trizole-3-thiol. Corros Sci 52:3331
Lukovits I, Lalman E, Zucchi F (2001) Corrosion inhibitors—correlation between electronic structure and efficiency. Corrosion 57:3–8
Özkır D, Kayakırılmaz K, Bayol E, Gürten A, Kandemirli A (2012) The inhibition effect of Azure A on mild steel in 1 M HCl. A complete study: adsorption, temperature, duration and quantum chemical aspects. Corros Sci 56:143–152
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981–2992
Amin MA, Khaled KF, Fadllalah SA (2010) Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions—experimental and theoretical studies. Corros Sci 52:140
Acknowledgements
The authors would like to thank University of Diyala, Iraq and Saratov State University, Russia for continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Rights and permissions
About this article
Cite this article
Mahmmod, A.A., Kazarinov, I.A., Khadom, A.A. et al. Experimental and Theoretical Studies of Mild Steel Corrosion Inhibition in Phosphoric Acid Using Tetrazoles Derivatives. J Bio Tribo Corros 4, 58 (2018). https://doi.org/10.1007/s40735-018-0171-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0171-y