Abstract
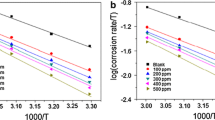
The effect of Holoptelea integrifolia leaf extract (HILE) on corrosive dissolution of mild steel in 1 M hydrochloric acid was investigated using weight loss, surface, electrochemical, and DFT methods. Results showed that corrosion protection capability of HILE increases with increasing its concentration and maximum value of 93.91% was attained at 400 mg/L concentration. Adsorption of phytochemicals present in HILE on metallic surface followed the Langmuir adsorption isotherm. Tafel polarization study revealed that HILE acts as mixed type inhibitor. EIS study revealed that HILE inhibits mild steel corrosion by getting adhered on the mild steel surface. SEM and AFM analyses showed that in presence of HILE, metallic surface becomes smooth because of the formation of inhibitive film over metallic surface. DFT calculations were also performed on the several active phytochemicals. It was found that β-amyrin was the most effective phytochemical for mild steel corrosion among the available constituents present in the extract.
Graphical Abstract










Similar content being viewed by others
References
Mai W, Soghrati S, Buchheit RG (2016) A phase field model for simulating the pitting corrosion. Corros Sci 110:157–166
Kıcır N, Tansuğ G, Erbil M, Tüken T (2016) Investigation of ammonium (2, 4-dimethylphenyl)-dithiocarbamate as a new, effective corrosion inhibitor for mild steel. Corros Sci 105:88–99
Gupta RK, Malviya M, Verma C, Quraishi M (2017) Aminoazobenzene and diaminoazobenzene functionalized graphene oxides as novel class of corrosion inhibitors for mild steel: experimental and DFT studies. Mater Chem Phys 198:360–373
Alibakhshi E, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B, Mahdavian M, Motamedi M (2018) Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Mol Liq 255:185–198
Zhang F, Tang Y, Cao Z, Jing W, Wu Z, Chen Y (2012) Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid. Corros Sci 61:1–9
Ehsani A, Mahjani M, Hosseini M, Safari R, Moshrefi R, Shiri HM (2017) Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J Colloid Interface Sci 490:444–451
Liu F, Zhang L, Yan X, Lu X, Gao Y, Zhao C (2015) Effect of diesel on corrosion inhibitors and application of bio-enzyme corrosion inhibitors in the laboratory cooling water system. Corros Sci 93:293–300
Sin HLY, Rahim AA, Gan CY, Saad B, Salleh MI, Umeda M (2017) Aquilaria subintergra leaves extracts as sustainable mild steel corrosion inhibitors in HCl. Measurement 109:334–345
Ramezanzadeh M, Sanaei Z, Bahlakeh G, Ramezanzadeh B (2018) Highly effective inhibition of mild steel corrosion in 3.5% NaCl solution by green Nettle leaves extract and synergistic effect of eco-friendly cerium nitrate additive: experimental, MD simulation and QM investigations. J Mol Liq 256:67–83
Li X, Xie X, Deng S, Du G (2014) Two phenylpyrimidine derivatives as new corrosion inhibitors for cold rolled steel in hydrochloric acid solution. Corros Sci 87:27–39
Guo L, Zhu S, Zhang S, He Q, Li W (2014) Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros Sci 87:366–375
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341
Mohammadinejad R, Karimi S, Iravani S, Varma RS (2016) Plant-derived nanostructures: types and applications. Green Chem 18:20–52
Varma RS (2014) Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem 16:2027–2041
Jeon H, Lim C, Lee JM, Kim S (2015) Chemical assay-guided natural product isolation via solid-supported chemodosimetric fluorescent probe. Chem Sci 6:2806–2811
Namboodiri VV, Varma RS (2002) Solvent-free sonochemical preparation of ionic liquids. Org Lett 4:3161–3163
Singh MS, Chowdhury S (2012) Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis. RSC Adv 2:4547–4592
Cioc RC, Ruijter E, Orru RV (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16:2958–2975
Gece G, Drugs (2011) A review of promising novel corrosion inhibitors. Corros Sci 53:3873–3898
Yesudass S, Olasunkanmi LO, Bahadur I, Kabanda MM, Obot I, Ebenso EE (2016) Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium. J Taiwan Inst Chem Eng 64:252–268
Diamanti MV, Velardi UV, Brenna A, Mele A, Pedeferri M, Ormellese M (2016) Compatibility of imidazolium-based ionic liquids for CO2 capture with steel alloys: a corrosion perspective. Electrochim Acta 192:414–421
Lozano I, Mazario E, Olivares-Xometl C, Likhanova N, Herrasti P (2014) Corrosion behaviour of API 5LX52 steel in HCl and H2SO4 media in the presence of 1, 3-dibencilimidazolio acetate and 1, 3-dibencilimidazolio dodecanoate ionic liquids as inhibitors. Mater Chem Phys 147:191–197
Muthukrishnan P, Jeyaprabha B, Prakash P (2017) Adsorption and corrosion inhibiting behavior of Lannea coromandelica leaf extract on mild steel corrosion. Arab J Chem 10:S2343–S2354
Hassan KH, Khadom AA, Kurshed NH (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Gerengi H, Uygur I, Solomon M, Yildiz M, Goksu H (2016) Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain Chem Pharm 4:57–66
Loto RT, Loto CA, Joseph O, Olanrewaju G (2016) Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results Phys 6:305–314
Dehdab M, Yavari Z, Darijani M, Bargahi A (2016) The inhibition of carbon-steel corrosion in seawater by streptomycin and tetracycline antibiotics: an experimental and theoretical study. Desalination 400:7–17
Madkour LH, Kaya S, Kaya C, Guo L (2016) Quantum chemical calculations, molecular dynamics simulation and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 1: mono-azo dye derivatives. J Taiwan Inst Chem Eng 68:461–480
Tezeghdenti M, Dhouibi L, Etteyeb N (2015) Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of Eucalyptus globulus leaves cultivated in Tunisia Arid zones. J Bio Tribo-Corros 1:16
Khadraoui A, Khelifa A, Hamitouche H, Mehdaoui R (2014) Inhibitive effect by extract of Mentha rotundifolia leaves on the corrosion of steel in 1 M HCl solution. Res Chem Intermed 40:961–972
Sutar RC, Kasture SB, Kalaichelvan V (2014) Evaluation of antidepressant activity of leaf extracts of Holoptelea integri Folia (roxb) planch in experimental animals. Int J Pharm Pharm Sci 6:250–253
Reddy MJ, Verma CB, Ebenso E, Singh K, Quaraishi M (2014) Electrochemical and thermodynamic investigation of nitrofurantoin as effective corrosion inhibitor for mild steel in 1M hydrochloric acid solution. Int J Electrochem Sci 9:4884–4899
Verma C, Quraishi M, Kluza K, Makowska-Janusik M, Olasunkanmi LO, Ebenso EE (2017) Corrosion inhibition of mild steel in 1M HCl by D-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci Rep 7:44432
Kumar D, Kumar K, Gupta J, Bishnoi N, Kumar S (2012) A mini review on chemistry and biology of Holoptelea integrifolia Roxb. Planch (Ulmaceae). Asian Pac J Trop Biomed 2:S1200–S1205
Aadesh U (2010) Anti-inflammatory evaluation of ethanolic extract of leaves of Holoptelea integrifolia, Planch. Ann Biol Res 1:185–195
Martinez S (2003) Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater Chem Phys 77:97–102
Yadav M, Kumar S, Purkait T, Olasunkanmi L, Bahadur I, Ebenso E (2016) Electrochemical, thermodynamic and quantum chemical studies of synthesized benzimidazole derivatives as corrosion inhibitors for N80 steel in hydrochloric acid. J Mol Liq 213:122–138
Olasunkanmi LO, Kabanda MM, Ebenso EE (2016) Quinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: electrochemical and quantum chemical studies. Physica E 76:109–126
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Verma C, Quraishi M, Singh A (2015) 2-Amino-5-nitro-4, 6-diarylcyclohex-1-ene-1, 3, 3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical studies. J Mol Liq 212:804–812
Verma C, Quraishi M, Singh A (2016) 5-Substituted 1H-tetrazoles as effective corrosion inhibitors for mild steel in 1 M hydrochloric acid. J Taibah Univ Sci 10:718–733
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mol Liq 258:89–97
Al-Moubaraki AH, Al-Howiti AA, Al-Dailami MM, Al-Ghamdi EA (2017) Role of aqueous extract of celery (Apium graveolens L.) seeds against the corrosion of aluminium/sodium hydroxide systems. J Environ Chem Eng 5:4194–4205
Khadom AA, Abd AN, Ahmed NA (2018) Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: kinetics and mathematical studies. S Afr J Chem Eng 25:13–21
Liao LL, Mo S, Luo HQ, Li NB (2017) Longan seed and peel as environmentally friendly corrosion inhibitor for mild steel in acid solution: experimental and theoretical studies. J Colloid Interface Sci 499:110–119
Alvarez PE, Fiori-Bimbi MV, Neske A, Brandán SA, Gervasi CA (2018) Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J Ind Eng Chem 58:92–99
Gupta NK, Quraishi M, Verma C, Mukherjee A (2016) Green Schiff’s bases as corrosion inhibitors for mild steel in 1 M HCl solution: experimental and theoretical approach. RSC Adv 6:102076–102087
Chauhan L, Gunasekaran G (2007) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49:1143–1161
Verma C, Quraishi M, Ebenso E, Obot I, El A, Assyry (2016) 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1M HCl: experimental and theoretical studies. J Mol Liq 219:647–660
Solmaz R, Kardaş G, Culha M, Erbil M (2008) Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim Acta 53:5941–5952
Solmaz R, Kardaş G, Erbil M (2008) Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1, 3, 4-thiadiazole on mild steel in hydrochloric acid media. Colloids Surf A 312:7–17
Lebrini M, Robert F, Lecante A, Roos C (2011) Corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros Sci 53:687–695
Verma C, Quraishi M, Singh A (2016) A thermodynamical, electrochemical, theoretical and surface investigation of diheteroaryl thioethers as effective corrosion inhibitors for mild steel in 1 M HCl. J Taiwan Inst Chem Eng 58:127–140
Verma C, Olasunkanmi LO, Ebenso EE, Quraishi MA, Obot IB (2016) Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120:11598–11611
Acknowledgements
Chandrabhan Verma gratefully acknowledges North-West University, Mafikeng, South Africa for providing financial support to the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, C., Quraishi, M.A., Ebenso, E.E. et al. A Green and Sustainable Approach for Mild Steel Acidic Corrosion Inhibition Using Leaves Extract: Experimental and DFT Studies. J Bio Tribo Corros 4, 33 (2018). https://doi.org/10.1007/s40735-018-0150-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0150-3