Abstract
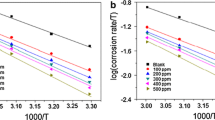
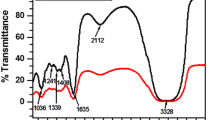
The effect of temperature and acid concentration on a newly formulated tannin as a corrosion inhibitor for carbon steel in oil and gas facilities was investigated. Corrosion rate of carbon steel in HCl acid solutions (0, 5, 10, 15, 20, 25 and 30%) by Rhizophora mucronata tannin (RMT) was studied using chemical (weight loss method) and spectroscopic (FTIR) techniques at various temperatures in the ranges of 26–70 °C. At 20% optimum concentration of acid, the FTIR result showed the presence of hydroxyl group, aromatic group, esters and the substituted benzene group, indicating the purity of the tannin. The increase in HCl acid concentration and temperature increased the corrosion rate, but the rate of corrosion was mild with RMT. Therefore, the use of RMT as a cost-effective and environmental-friendly corrosion-inhibiting agent for carbon steel is herein proposed.











Similar content being viewed by others
References
Ituen E, Akaranta O, James A (2016) Green anticorrosive oil field chemical from 5-hydroxytryptophan and synergistic additives for X80 steel surface protection in acidic oil well treatment fluid. J Mol Liq 224:408–419
Deyab M (2007) Effect of cationic surfactant and inorganic anions on the electrochemical behaviour of carbon steel in formation water. Corros Sci 49:2315
Cheng S, Chen S, Liu T, Chang X, Yin Y (2007) Carboxylmethylchitosan +Cu2+ Mixture as an inhibitor used for mild steel in 1 M HCl. Electrochem Acta 52(19):5932–5938
Daoud D, Daoud T, Issaadi S, Chafaa S (2014) Adsorption and corrosion inhibition of new synthesized thiophene schiff base on mild steel X52 in HCl and H2SO4 solution. Corros Sci 79:50
Berger MN, Boocock G, Harward RN (1969) Polymerization of olefins by ziegler catalysts. Adv Catal 19:211–240
Daminov A, Ragulin V (2006). Mechanical formation of corrosion damage of inter equipment in wells by continuous scale-inhibitor dosing utilizing surface dosing system: testing scale and corrosion inhibitor. Paper SPE-100476 presented at the SPE international oil field corrosion symposium. 30th May
Grassino A, Halambek J, Djakovic S, Brncic S (2015) Utilization of tomatoes peel waste from canning factory as a potential source of pectin production and application as a tin corrosion inhibitor. Food Hydrocoll 52:265–274
Chauhan L, Gunasekaran G (2006) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49:1143–1161
Okafor PC, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllantus amarus extract on the corrosion of mild steel in acidic media. Corros Sci 50:2310–2317
Oguzie E (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50:2993–2998
Shyamala M, Arulanantham A (2008) Eclipta alba as corrosion pickering inhibitor on mild steel in hydrochloric acid. J Mater Sci Technol 25(5):633–636
Ostovari A, Hoseinieh S, Peikari M, Shadizadeh S, Hashemi S (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition of henna and its constituents. Corros Sci 51:1935–1941
Satapathy A, Gunasekaran G, Sahoo S, Amit K, Rodrigues P (2009) Corrosion Inhibition of Justicia gendarussa Plant Extract in Hydrochloric Acid Solution. Corros Sci 51:2848–2856
Umoren S, Eduok U, Solomon M, Udoh A (2011) Corrosion inhibition by leaves and stem extract of sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic technique. Arab J Chem 9:S209–S224
Al-Sahlanee H, Sultan A, Al-Faize M (2013) Corrosion inhibition of carbon steel in 1 M HCl solution using Sesbania sesban extract. Aquat Sci Technol 1(2):135–151
Arockiasamy P, Sheela X, Thenmozhi G, Franco M, Sahayaraj J, Santhi R (2014). Evaluation of corrosion inhibition of mild steel in 1 M hydrochloric acid solution by Mollugo cerviana. Int J Corros 1–7
Hussin M, Kassim M, Razali N, Dahon N, Nasshorudin D (2011) The effect of tinospora crispa extract as a natural mild steel corrosion inhibitor in 1 M HCl. Mater Chem Phys 125:461–468
Hussin M, Rahim A, Ibrahim M, Brosse N (2015) The capacity of ultra filtered alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as a green corrosion inhibitor for mild steel in 05 M HCl solution. Measurement 78:90–103
Aribo S, Olusegun S, Ibhadiyi L, Oyetunji A, Folorunso D (2016) Green inhibitor for corrosion protection in acidizing oilfield environment. J Assoc Arab Univ Basic Appl, Sci
El-Etre AY, Ali AI (2017) A novel new inhibitor for C-steel corrosion in 2.0 mol. L−1 hydrochloric acid solution. Chin J Chem Eng 25:373–380
Raghavendra N, Bhat J (2017) Chemical and electrochemical studies on the areca fat as a novel and sustainable corrosion inhibitor for industrially important material in hostile fluid environments. J Bio Tribo Corros 3:12
Martinez S, Stagljar I (2003) Correlation between the molecular and the corrosion inhibition efficiency of chestnut tannin in acidic solution. J Mol Struct Theochem 640:167–174
Rahim A, Rocca E, Steinmetz J, Ibrahim M (2007) Mangrove tannins and their flavoured monomers as alternative steel corrosion inhibitors in acid medium. Corros Sci 49:402–417
Rahim A, Kassim M, Rocca E, Steinmetz J (2011) Mangrove (Rhizophora apiculata) tannin: an eco-friendly rust converter. Corros Eng, Sci Technol 46(4):425–431
Shah A, Rahim A, Yahya S, Raja B (2011) Acid corrosion inhibition of copper by mangrove tannin. Pigment Resin Technol 40(2):118–122
Shah A, Rahim A, Hamid S, Yahya M (2013) Green inhibitor for copper corrosion by mangrove tannin. Int J Electrochem Sci 8:2140–2153
Nik WB, Hajar HM, Idora M, Suriani M, Yakubi A (2015) Effect of mangrove bark condensed tannins (Rhizophora apiculata) as corrosion inhibitor for mild steel in simulated splash zone. J Sci Res Dev 2(13):59–63
Peres R, Cassel E, Azambuja S (2012). Black wattle tannin as steel corrosion inhibitor. Int Sch Res Netw 9
Oki M, Charles E, Alaka C, Oki T (2011) Corrosion inhibition of mild steel in hydrochloric acid by tannins from Rhizophora racemosa. Mater Sci Appl 2:592–595
Brown A, Ko H (1997) Black wattle and its utilization. Rural Industrial Research and Development Cooperation, Barton
Mabrour J, Aksirra M, Azzi M (2004) Effect of vegetal tannin on anodic copper dissolution in chloride solution. Corros Sci 46(8):1833–1846
Yahya S, Shah A, Rahim A, Aziz A, Roslan R (2008) Phase transformation of rust in the presence of various tannin. J Phys Sci 19(1):31–41
Rahim A, Rocca E, Steinmetz J, Kassim M (2008) Inhibitive action of mangrove tannins and phosphoric acid on pre-rusted steel via electrochemical methods. Corros Sci 50:1546–1550
Hill DG, Romijn H (2000) Reduction of risk to the marine environment from oil field chemicals environmentally improved acid corrosion inhibition for well stimulation. NACE International Corrosion Paper No.00342, Orlando, Florida, 26–31 March
Smith C, Dollarhide F, Byth M (1978) Acid corrosion inhibitor: are we getting what we need? J Petrol Technol 30:737–746
Loo AY, Jain K, Darah I (2008) Antioxidant activity of compound isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem 107:1151–1160
Horsup DI, Clark JC, Binks BP, Fletcher PD, Hicks JT (2010) The faith of oil field corrosion inhibitors in multiphase system. Corrosion 66 (3)
Finsgar M, Jackson J (2014) Application of corrosion inhibitor for steel in acidic media for oil and gas industry: a review. Corros Sci 86:17–41
Papavinasam S, Revie R, Attard M, Demoz A, Michaelian K (2003) Comparison of technique for monitoring. Corrosion 59:1096–1111
Khadom A, Yaro A, Kadum A (2009) The effect of temperature and acid concentration on corrosion of mild steel in hydrochloric acid medium. Am J Appl Sci 6(7):1403–1409
Emran K (2013) Effect of concentration and temperature on the corrosion properties of Fe–Ni–Mn Alloy in HCl solutions. Res Chem Intermed 41:3583–3596
Haynes GS, Baboian R (1990) Review of laboratory corrosion test and standards. Phila ASTM Spec Tech Publ 1000:505–509
Gust J (1991) Application of infrared spectroscopy for investigation of rust phase component conversion by agents containing oak tannin and phosphoric acid. Corrosion 47(6):453–457
Hoong Y, Paridaha M, Luqman C, Koh M, Loh Y (2009) Fortification of sulfited tannin from the bark of Acacia mangium with phenol-formaldehyde for use as plywood adhesive. Ind Crops Prod 30:416–421
Socrates G (2001) Infrared and Raman characteristics group frequency: tables and charts. Wiley, Chichester
Hinggins RA (2004) Engineering metallurgy applied physical metallurgy. Vinod Vasishtha for Viva Books Private Limited, New Delhi
Ogundare O, Momoh I, Akinribide O, Adetunji A, Borode J, Olusunle S, Adewoye O (2012) Comparative study of corrosion sensitivity of ferrous metals in crude oil. J Miner Mater Charact Eng 11(6):559–568
Abdul A, Azim Sanad S (1972) Effect of acid concentration, C-content and temperature on the corrosion rate of steel in HCl. Corros Sci 12:313–324
Abiola OK (2005) Adsorption of methionine on mild steel. J Chil Chem Soc 50:685–690
Umoren S, Ogbobe O, Igwe I, Ebenso E (2008) Inhibition of mild steel corrosion in acidic medium using synthetic and natural occurring polymers and synthetic halide additives. Corros Sci 50:1998–2006
Umoren S, Solomon S, Eduok U, Obot I, Israel A (2014) Inhibition of mild steel corrosion in H2SO4 solution by coconut coir dust extract obtained from different solvent systems and synergistic effect of iodine ions: ethanol and acetone extracts. J Environ Chem Eng 2:1048–1060
Acknowledgement
The authors would like to thank the Ministry of Higher Education (MOHE), Malaysia, and Universiti Teknologi Malaysia (UTM), for supporting this research through Research Management Grant Vot. No. Q. J30000.2546.14H50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Agi, A., Junin, R., Zakariah, M.I. et al. Effect of Temperature and Acid Concentration on Rhizophora mucronata Tannin as a Corrosion Inhibitor. J Bio Tribo Corros 4, 5 (2018). https://doi.org/10.1007/s40735-017-0121-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-017-0121-0