Abstract
Bio-based lubricants have gained prominence over conventional petroleum-based oils, progressively over the last two decades as biolubricants. This trend is observed in almost every industry that has been dependent on lubricants and oils irrespective of their applications. Factors that initiated and fueled this trend vary from stringent government regulations over petroleum-based oils to the high passed depletion of oil reserves. But the most concerning factor that has fast-tracked the need for biolubricants is the toxic and harmful effect of used petroleum oils has on the environment and ecological factors. It is estimated that nearly 50% of all lubricants produced are introduced to the environment which has spurred the interest in biolubricants. This review discusses various types of eco-friendly bio-lubrications that will become a sustainable and economical alternative to the conventional petroleum-based lubricants by being sourced from renewable resources. Biolubricants are seen to be feasible and versatile lubricants with higher lubricity, lower volatility, higher shear stability, higher viscosity index, higher load-carrying capacity, and superior detergency and dispersancy when compared to petroleum-based lubricants. The review also investigates in detail the poor thermal-oxidative stability, biological deterioration, their poor solidification at low temperatures, and hydrolytic instability as well as mechanical and chemical enhancements that seek to rectify these issues. Furthermore, economical and legislative landscape of biolubricants is discussed.












Similar content being viewed by others
References
Menezes PL, Ingole SP, Nosonovsky M, Kailas SV, Lovell MR (2013) Tribology for scientists and engineers. Springer, New York
Naegely PC (1993) Environmentally acceptable lubricants, in seed oils for the future. AOCS Press, Champaign
Mang T, Dresel W (2006) Lubricants and lubrication. Wiley, Weinheim
Schneider MP (2006) Plant-oil-based lubricants and hydraulic fluids. J Sci Food Agric 86(12):1769–1780
IENICA (2004) Biolubricants: Market Data Sheet. http://www.ienica.net/marketdatasheets/biolubricantsmds.pdf
Holmberg K, Andersson P, Nylund N-O, Mäkelä K, Erdemir A (2014) Global energy consumption due to friction in trucks and buses. Tribol Int 78:94–114. doi:10.1016/j.triboint.2014.05.004
Salimon J, Salih N, Yousif E (2010) Biolubricants: raw materials, chemical modifications and environmental benefits. Eur J Lipid Sci Technol 112(5):519–530
Aluyor EO, Obahiagbon KO, Ori-jesu M (2009) Biodegradation of vegetable oils: a review. Sci Res Essays 4(6):543–548
Deffeyes KS (2009) Hubbert’s peak. Princeton University Press, Princeton
Goodstein DL (2004) Out of gas: the end of the age of oil. W.W. Norton, New York
Lovell M, Higgs CF, Deshmukh P, Mobley A (2006) Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J Mater Process Technol 177(1):87
Li W, Kong XH, Ruan M, Ma FM, Jiang YF, Liu MZ, Chen Y, Zuo XH (2010) Green waxes, adhesives and lubricants. Philos Trans R Soc A Math Phys Eng Sci 368(1929):4869–4890
Totten GE, Westbrook SR, Shah RJ (2003) Fuels and lubricants handbook technology, properties, performance, and testing. ASTM International, West Conshohocken
Backé W (1993) The present and future of fluid power. Proc Inst Mech Eng Part I 207(4):193–212
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod 28(1):1–10
Meier MAR, Metzger JO, Schubert US (2007) Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev 36(11):1788–1802
Feldmann DG, Remmelmann A (1996) Biologisch schnell abbaubare Hydraulikfluessigkeiten—Ergebnisse von Pruefstandstests und Folgerungen fuer die Anwendung. Aachener Fluidtechnisches Kolloquium 12(1):59–80
Feldmann DG, Kessler M (2002) Fluid qualification tests—evaluation of the lubricating properties of biodegradable fluids. Ind Lubr Tribol 54:117–129
Feldmann DG, Hinrichs J (1997) Evaluation of the lubrication properties of biodegradable fluids and their potential to replace mineral oil in heavily loaded hydrostatic transmissions. ASTM Spec Tech Publ 1310:220
Fessenbecker A, Korff J (1995) Additives for environmentally more friendly lubricant. J Jpn Soc Tribol 40(4):306
Korff J, Fessenbecker A (1993) Additives for biodegradable lubricants. NLGI Spokesm 57(3):19
Fox NJ, Tyrer B, Stachowiak GW (2004) Boundary lubrication performance of free fatty acids in sunflower oil. Tribol Lett 16(4):275–281
Grushcow J, Smith MA (2005) Next generation feedstocks from new frontiers in oilseed engineering. In: ASME conference proceedings 42010, pp 487–488
Grushcow J (2005) High oleic plant oils with hydroxy fatty acids for emission reduction. In: 2005 World Tribology Congress III. American Society of Mechanical Engineers, Washington, DC, pp 485–486
Lundgren SM, Ruths M, Danerlov K, Persson K (2008) Effects of unsaturation on film structure and friction of fatty acids in a model base oil. J Colloid Interface Sci 326(2):530–536
Hu Z-S, Hsu SM, Wang PS (1992) Tribochemical reaction of stearic acid on copper surface studied by surface enhanced Raman spectroscopy. Tribol Trans 35(3):417–422
Salih N, Salimon J, Yousif E (2011) The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind Crops Prod 34(1):1089–1096
Erhan SZ, Sharma BK, Perez JM (2006) Oxidation and low temperature stability of vegetable oil-based lubricants. Ind Crops Prod 24(3):292–299
Koshima H, Kamano H, Hisaeda Y, Liu H, Ye S (2010) Analyses of the adsorption structures of friction modifiers by means of quantitative structure-property relationship method and sum frequency generation spectroscopy. Tribol Online 5(3):165–172
Bowden FP, Leben L, Tabor D (1939) The influence of temperature on the stability of a mineral oil. Trans Faraday Soc 35:900–934
Lovell MR, Menezes PL, Kabir MA, Higgs CF III (2010) Influence of boric acid additive size on green lubricant performance. Philos Trans R Soc A Math Phys Eng Sci 368(1929):4851–4868
Bauccio, M., and American Society for, M. (1993) ASM metals reference book. ASM International, Materials Park
Deshmukh P, Lovell M, Sawyer WG, Mobley A (2006) On the friction and wear performance of boric acid lubricant combinations in extended duration operations. Wear 260(11–12):1295–1304
Menezes PL, Lovell MR, Kabir MA, Higgs CF III, Rohatgi PK (2012) Green lubricants: role of additive size. In: Nosonovsky M, Bhushan B (eds) Green tribology. Springer, Berlin, pp 265–286
Havet L, Blouet J, Robbe Valloire F, Brasseur E, Slomka D (2001) Tribological characteristics of some environmentally friendly lubricants. Wear 248(1–2):140–146
Santos ODH, Morais JM, Andrade FF, Aguiar TA, Rocha Filho PA (2011) Development of vegetable oil emulsions with lamellar liquid-crystalline structures. J Dispers Sci Technol 32(3):433–438
Reeves CJ, Menezes PL, Jen T-C, Lovell MR (2012) Evaluating the tribological performance of green liquid lubricants and powder additive based green liquid lubricants. In: STLE annual meeting & exhibition. STLE, St. Louis
Lundgren SM, Persson K, Mueller G, Kronberg B, Clarke J, Chtaib M, Claesson PM (2007) Unsaturated fatty acids in alkane solution: adsorption to steel surfaces. Langmuir 23(21):10598–10602
Reeves CJ, Menezes PL, Lovell MR, Jen T-C (2013) The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol Lett 51(3):437–452
Duzcukoglu H, Sahin O (2011) Investigation of wear performance of canola oil containing boric acid under boundary friction condition. Tribol Trans 54(1):57–61
Erdemir A (1990) Tribological properties of boric acid and boric acid forming surfaces: part 1. Crystal chemistry and self-lubricating mechanism of boric acid. In: Society of tribologists lubrication engineers annual conferrence. Argonne National Labs, Denver, CO, USA
Werman MJ, Neeman I (1987) Avocado oil production and chemical characteristics. J Am Oil Chem Soc 64(2):229–232
Nosonovsky M, Bhushan B (2012) Green tribology biomimetics, energy conservation and sustainability. Springer, Berlin
Bennion M, Scheule B (2010) Introductory foods. Prentice Hall, Upper Saddle River
Biermann U, Metzger JO (2008) Synthesis of alkyl-branched fatty acids. Eur J Lipid Sci Technol 110(9):805–811
Randles SJ (1994) Formulation of environmentally acceptable lubricants. In: 49th STLE annual meeting, Pittsburgh
Shubkin RL (1993) Synthetic lubricants and high-performance functional fluids. Dekker, New York
Uosukainen E, Linko YY, Lamsa M, Tervakangas T, Linko P (1998) Transesterification of trimethylolpropane and rapeseed oil methyl ester to environmentally acceptable lubricants. J Am Oil Chem Soc 75(11):1557–1563
Kodali DR (2003) Biobased lubricants—chemical modification of vegetable oils. INFORM 14:121–143
Wagner H, Luther R, Mang T (2002) Lubricant base fluids based on renewable raw materials—their catalytic manufacture and modification. Appl Catal A 221(1):429
Andersen MPS, Hurley MD, Wallington TJ, Blandini F, Jensen NR, Librando V, Hjorth J, Marchionni G, Avataneo M, Visca M (2004) Atmospheric chemistry of CH3O(CF2CF2O)nCH3 (n = 1-3): kinetics and mechanism of oxidation Initiated by Cl atoms and OH radicals, IR spectra, and global warming potentials. J Phys Chem A 108:1964–1972
Scientific Assessment of Ozone Depletion (2003) 2002, National Oceanic and Atmospheric Administration; National Aeronautics and Space Administration; United Nations Environment Programme; World Meteorological Organization; & European Commission, Washington, DC; Nairobi, Kenya; Geneva, Switzerland; Brussels, Belgium
Wallington TJ, Schneider WF, Sehested J, Bilde M, Platz J, Nielsen OJ, Christensen LK, Molina MJ, Molina LT, Wooldridge PW (1997) Atmospheric chemistry of HFE-7100 (C4F9OCH3): reaction with OH radicals, UV spectra and kinetic data for C4F9OCH2· and C4F9OCH2O2· radicals, and the atmospheric fate of C4F9OCH2O· radicals. J Phys Chem A 101(44):8264–8274
Marchionni G, Avataneo M, De Patto U, Maccone P, Pezzin G (2005) Physical properties of four α,ω-dimethoxyfluoropolyethers. J Fluor Chem 126(4):463–471
Marco A, Patto UD, Giuseppe M (2003) Liquid-liquid extraction of polar organic substances from their aqueous solutions with fluorinated extracting liquids. European Patent EP 1,346,757
Giuseppe, M., and Mario, V., 2003. Perfluoropolyethers (PFPEs) having at least an alkylether end group and respective preparation process. European Patent Application EP 1275678
Marchionni G, Petricci S, Guarda PA, Spataro G, Pezzin G (2004) The comparison of thermal stability of some hydrofluoroethers and hydrofluoropolyethers. J Fluor Chem 125(7):1081–1086
Marchionni G, Maccone P, Pezzin G (2002) Thermodynamic and other physical properties of several hydrofluoro-compounds. J Fluor Chem 118(1–2):149–155
Asadauskas S, Erhan S (1999) Depression of pour points of vegetable oils by blending with diluents used for biodegradable lubricants. J Am Oil Chem Soc 76(3):313–316
Reeves CJ, Garvey SL, Menezes PL, Dietz ML, Jen TC, Lovell MR (2012) Tribological performance of environmentally friendly ionic liquid lubricants. ASME/STLE 2012 international joint tribology conference. STLE, Denver, CO, USA
Bermúdez MD, Jiménez AE, Sanes J, Carrión FJ (2009) Ionic liquids as advanced lubricant fluids. Molecules 14(8):2888–2908
Canter N (2005) Evaluating ionic liquids as potential lubricants. Tribol Lubr Technol 61(9):15–17
Liu W, Ye C, Gong Q, Wang H, Wang P (2002) Tribological performance of room-temperature ionic liquids as lubricant. Tribol Lett 13(2):81–85
Zhou F, Liang Y, Liu W (2009) Ionic liquid lubricants: designed chemistry for engineering applications. Chem Soc Rev 38(9):2590–2599
Battez, HA, Alonso DB, Rodriguez RG, Viesca Rodriguez JL, Fernandez-Gonzalez A, Garrido AH (2011) Lubrication of DLC and tin coatings with two ionic Liquids used as neat lubricant and oil additives. In: Proceedings of the STLE/ASME international joint tribology conference—2011. American Society of Mechanical Engineers, Los Angeles, CA, USA
Freemantle M (2010) An introduction to ionic liquids. RSC Publication, Cambridge
Sheldon RA, Arends I, Hanefeld U (2007) Green chemistry and catalysis. Wiley, Weinheim
Yao M, Liang Y, Xia Y, Zhou F (2009) Bisimidazolium ionic liquids as the high-performance antiwear additives in poly(ethylene glycol) for steel-steel contacts. ACS Appl Mater Interfaces 1(2):467–471
Reeves CJ, Menezes PL, Garvey SL, Jen TC, Dietz ML, Lovell MR (2013) The effect of anion-cation moiety manipulation to characterize the tribological performance of environmentally benign room temperature ionic liquid lubricants. In: 2013 STLE annual meeting and exhibition (STLE2013). Society of Tribologists & Lubrication Engineers, Detroit, MI, USA
Reeves CJ, Menezes PL, Lovell MR, Jen TC, Garvey SL, Dietz ML (2013) The tribological performance of bio-based room temperature ionic liquid lubricants: A possible next step in biolubricant technology. In: 5th World tribology congress—Society of Tribologists & Lubrication Engineers, Torino, Italy
Espinosa T, Sanes J, Bermúdez M-D (2016) New alkylether-thiazolium room-temperature ionic liquid lubricants: surface interactions and tribological performance. ACS Appl Mater Interfaces 8(28):18631–18639
Itoga M, Aoki S, Suzuki A, Yoshida Y, Fujinami Y, Masuko M (2016) Toward resolving anxiety about the accelerated corrosive wear of steel lubricated with the fluorine-containing ionic liquids at elevated temperature. Tribol Int 93(Part B):640–650
Jiménez AE, Bermúdez MD, Iglesias P, Carrión FJ, Martínez-Nicolás G (2006) 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel–aluminium contacts. Wear 260(7–8):766–782
Somers AE, Howlett PC, Sun J, MacFarlane DR, Forsyth M (2010) Transition in wear performance for ionic liquid lubricants under increasing load. Tribol Lett 40(2):279–284
Minami I, Kita M, Kubo T, Nanao H, Mori S (2008) The tribological properties of ionic liquids composed of trifluorotris(pentafluoroethyl) phosphate as a hydrophobic anion. Tribol Lett 30(3):215–223
Espinosa T, Sanes J, Jiménez A-E, Bermúdez M-D (2013) Surface interactions, corrosion processes and lubricating performance of protic and aprotic ionic liquids with OFHC copper. Appl Surf Sci 273:578–597
Saurín N, Minami I, Sanes J, Bermúdez MD (2016) Study of the effect of tribo-materials and surface finish on the lubricant performance of new halogen-free room temperature ionic liquids. Appl Surf Sci 366:464–474
Quijano G, Couvert A, Amrane A, Darracq G, Couriol C, Le Cloirec P, Paquin L, Carrie D (2011) Toxicity and biodegradability of ionic liquids: new perspectives towards whole-cell biotechnological applications. Chem Eng J 174(1):27–32
Gathergood N, Scammells PJ, Garcia MT (2006) Biodegradable ionic liquids, part III. The first readily biodegradable ionic liquids. Green Chem 8(2):156–160
Wu M, Navarrini W, Spataro G, Venturini F, Sansotera M (2012) An environmentally friendly class of fluoropolyether: alpha-omega-dialkoxyfluoropolyethers. Appl Sci 2(2):351–367
Atefi F, Garcia MT, Singer RD, Scammells PJ (2009) Phosphonium ionic liquids: design, synthesis and evaluation of biodegradability. Green Chem 11(10):1595–1604
Omotowa BA, Phillips BS, Zabinski JS, Shreeve JM (2004) Phosphazene-based ionic liquids: synthesis, temperature-dependent viscosity, and effect as additives in water lubrication of silicon nitride ceramics. Inorg Chem 43(17):5466–5471
Freire MG, Carvalho PJ, Gardas RL, Marrucho IM, Santos LM, Coutinho JA (2008) Mutual solubilities of water and the [C(n)mim][Tf(2)N] hydrophobic ionic liquids. J Phys Chem B 112(6):1604–1610
Smith SA, King RE, Min DB (2007) Oxidative and thermal stabilities of genetically modified high oleic sunflower oil. Food Chem 102(4):1208–1213
Marmesat S, Morales A, Velasco J, Carmen Dobarganes M (2012) Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying. Food Chem 135(4):2333–2339
Fox NJ, Stachowiak GW (2007) Vegetable oil-based lubricants: a review of oxidation. Tribol Int 40(7):1035–1046
Jayadas NH, Nair KP (2006) Coconut oil as base oil for industrial lubricants—evaluation and modification of thermal, oxidative and low temperature properties. Tribol Int 39(9):873–878
Zeman A, Sprengel A, Niedermeier D, Späth M (1995) Biodegradable lubricants studies on thermo-oxidation of metal-working and hydraulic fluids by differential scanning calorimetry (DSC). Thermochim Acta 268:9–15
Mendoza G, Igartua A, Fernandez-Diaz B, Urquiola F, Vivanco S, Arguizoniz R (2011) Vegetable oils as hydraulic fluids for agricultural applications. Grasas Aceites 62(1):29–38
Rudnick LR, Shubkin RL (1999) Synthetic lubricants and high-performance functional fluids. Marcel Dekker, New York
Eisentraeger A, Schmidt M, Murrenhoff H, Dott W, Hahn S (2002) Biodegradability testing of synthetic ester lubricants–effects of additives and usage. Chemosphere 48(1):89–96
Birova A, Pavlovicov A, Cvenros J (2002) Lubricating oils based on chemically modified vegetable oils. J Synth Lubr 18(4):291–299
King JW, Holliday RL, List GR, Snyder JM (2001) Hydrogenation of vegetable oils using mixtures of supercritical carbon dioxide and hydrogen. J Am Oil Chem Soc 78(2):107–113
Erhan SZ, Adhvaryu A, Sharma BK (2006) Chemically functionalized vegetable oils. Chem Ind 111:361–388
Doll KM, Sharma BK, Erhan SZ (2007) Synthesis of branched methyl hydroxy stearates including an ester from bio-based levulinic acid. Ind Eng Chem Res 46(11):3513–3519
Yunus R, Fakhrul-Razi A, Ooi TL, Iyuke SE, Perez JM (2004) Lubrication properties of trimethylolpropane esters based on palm oil and palm kernel oils. Eur J Lipid Sci Technol 106:52–60
Hwang H-S, Adhvaryu A, Erhan SZ (2003) Preparation and properties of lubricant base stocks from epoxidized soybean oil and 2-ethylhexanol. J Am Oil Chem Soc 80(8):811–815
Verkuijlen E, Kapteijn F, Mol JC, Boelhouwer C (1977) Heterogeneous metathesis of unsaturated fatty acid esters. J Chem Soc 1(7):198–199
Schmidt MA, Dietrich CR, Cahoon EB (2006) Biotechnological enhancement of soybean oil for lubricant applications. Chem Ind 111:389–398
Holser R, Doll K, Erhan S (2006) Metathesis of methyl soyate with ruthenium catalysts. Fuel 85(3):393–395
Erhan SZ, Bagby MO, Nelsen TC (1997) Drying properties of metathesized soybean oil. J Am Oil Chem Soc 74(6):703–706
Saad B, Wai WT, Lim BP (2008) Comparative study on oxidative decomposition behavior of vegetable oils and its correlation with iodine value using thermogravimetric analysis. J Oleo Sci 57(4):257–261
Yanishlieva NV, Marinova EM (2001) Stabilisation of edible oils with natural antioxidants. Eur J Lipid Sci Technol 103:752–767
Kapilan N, Reddy RP, Ashok Babu TP (2009) Technical aspects of biodiesel and its oxidation stability. Int J ChemTech Res 1(2):278–282
Domingos AK, Saad EB, Vechiatto WWD, Wilhelm HM, Ramos LP (2007) The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). Braz Chem Soc 18(2):416–423
International Organization for, S. (2006) Animal and vegetable fats and oils: determination of oxidative stability (accelerated oxidation test). International Organization for Standardization, Geneva
Gertz C, Klostermann S, Kochhar SP (2000) Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Hu X (2005) On the size effect of molybdenum disulfide particles on tribological performance. Ind Lubr Tribol 57(6):255–259
Huang HD, Tu JP, Gan LP, Li CZ (2006) An investigation on tribological properties of graphite nanosheets as oil additive. Wear 261(2):140–144
Sunqing Q, Junxiu D, Guoxu C (1999) A review of ultrafine particles as antiwear additives and friction modifiers in lubricating oils. Lubr Sci 11(3):217–226
Xiaodong Z, Xun F, Huaqiang S, Zhengshui H (2007) Lubricating properties of Cyanex 302-modified MoS2 microspheres in base oil 500SN. Lubr Sci 19(1):71–79
Düzcükoğlu H, Acaroğlu M (2010) Lubrication properties of vegetable oils combined with boric acid and determination of their effects on wear. Energy Sources Part A 32(3):275–285
Erdemir A, Bindal C, Fenske GR (1996) Formation of ultralow friction surface films on boron carbide. Appl Phys Lett 68(12):1637–1639
Erdemir A (1997) Preparation of ultralow-friction surface films on vanadium diboride. Wear 205(1–2):236–239
Erdemir A, Eryilmaz OL, Fenske GR (1999) Self-replenishing solid lubricant films on boron carbide. Surf Eng 15(4):291–295
Erdemir A, Fenske GR, Nichols FA, Erck RA, Busch DE (1990) Self-lubricating boric acid films for tribological applications. In: Japan international tribology conference, Nagoya, Japan. Argonne National Lab, Lemont
Erdemir A, University of, Chicago (1995) Lubrication from mixture of boric acid with oils and greases. US Patent 5,431,830
Streitwieser A, Heathcock CH, Kosower EM (1992) Introduction to organic chemistry. Prentice Hall, Upper Saddle River
Canter N (2001) It isn’t easy being green-the promise, perils, and progress of environmentally friendly lubricants. Lubr World 9(3):16–21
Salimon J, Salih N, Yousif E (2012) Improvement of pour point and oxidative stability of synthetic ester base stocks for biolubricant applications. Arab J Chem 5(2):193–200
Sharma BK, Stipanovic AJ (2003) Development of a new oxidation stability test method for lubricating oils using high-pressure differential scanning calorimetry. Thermochim Acta 402(1–2):1–18
Gapinski, R. E., Joseph, I. E., Layzell, B. D., and Society of Automotive, E. (1994) A vegetable oil based tractor lubricant. Society of Automotive Engineers, Warrendale
Becker R, Knorr A (1996) An evaluation of antioxidants for vegetable oils at elevated temperatures. Lubr Sci 8(2):95–117
Ohkawa S, Konishi A, Hatano H, Ishihama K (1996) Oxidation and corrosion characteristics of vegetable-base biodegradable hydraulic oils. SAE Trans 104(4):737
Salimon J, Salih N (2009) Oleic acid diesters: synthesis, characterization and low temperature properties. Eur J Sci Res 32(2):216–222
Salimon J, Salih N (2009) Preparation and characteristic of 9, 10-epoxyoleic acid α-hydroxy ester derivatives as biolubricant base oil. Eur J Sci Res 31(2):265–272
Salimon J, Salih N (2009) Improved low temperature properties of 2-ethylhexyl 9(10)-hydroxy-10(9)-acyloxystearate derivatives. Eur J Sci Res 31(4):583–591
Salimon J, Salih N (2009) Substituted esters of octadecanoic acid as potential biolubricants. Eur J Sci Res 31(2):273–279
Biermann U, Friedt W, Lang S, Lühs W, Machmüller G, Metzger UO, Klaas MR, Schäfer HJ, Schneider MP (2008) New syntheses with oils and fats as renewable raw materials for the chemical industry. In: Kamm B, Gruber PR, Kamm M (eds) Biorefineries—industrial processes and products. Wiley, Weinheim, pp 253–289
Salimon J, Salih N, Yousif E (2011) Chemically modified biolubricant base stocks from epoxidized oleic acid: improved low temperature properties and oxidative stability. J Saudi Chem Soc 15(3):195–201
Biermann U, Metzger JO (1999) Friedel-Crafts alkylation of alkenes: ethylaluminum sesquichloride induced alkylations with alkyl chloroformates. Angew Chem Int Ed Engl 38:3675–3677
Metzger JO, Riedner U (1989) Free radical additions to unsaturated fatty acids. Fat Sci Technol 91:18–23
Metzger JO, Linker U (1991) New results of free radical additions to unsaturated fatty compounds. Lipid 93(7):244–249
Metzger JO, Biermann U (1993) Alkylaluminium dichloride induced Friedel-Crafts acylation of unsaturated carboxylic acids and alcohols. Liebigs Ann Chem 1993(6):645–650
Biermann U, Metzger JO (1991) Lewis acid catalyzed additions to unsaturated fatty compounds, II: alkylaluminium halide catalyzed ene reactions of unsaturated fatty compounds and formaldehyde. Lipid 93(8):282–284
Pryde EH (1984) Hydroformylation of unsaturated fatty acids. J Am Oil Chem Soc 61(2):419–425
Frankel E, Pryde E (1977) Catalytic hydroformylation and hydrocarboxylation of unsaturated fatty compounds. J Am Oil Chem Soc 54(11):A873–A881
Xia Z, Kloeckner U, Fell B (1996) Hydroformylation of mono and multiple unsaturated: fatty substances with heterogenized cobalt carbonyl and rhodium carbonyl catalysts. Lipid 98:313–321
Behr A, Laufenberg A (1991) Synthesis of new branched fatty acids by rhodium catalyzed homogeneous oligomerization. Fat Sci Technol 93:20–24
Henkel K (1991) Al, Dusseldorf. Germany Patent
Keller U, Fischer J, Hoelderich WF (2000) New lubricants from renewable resources: ecotoxicities and oxidative characteristics. Oelhydraulik und Pneumatik 4:240–245
Crivello JV, Fan MX (1992) Catalysis of ring-opening and vinyl polymerizations by dicobaltoctacarbonyl. J Polym Sci Polym Chem 30(1):31–39
Isbell TA (2011) Chemistry and physical properties of estolides. Grasas Aceites Grasas y Aceites 62(1):8–20
Brimberg UI, Kamal-Eldin A (2003) On the kinetics of the autoxidation of fats: influence of pro-oxidants, antioxidants and synergists. Eur J Lipid Sci Technol 105(2):83–91
Gordon MH, Kourimska L (1995) The effects of antioxidants on changes in oils during heating and deep frying. J Sci Food Agric 68(3):347–353
Schober S, Mittellbach M (2004) The impact of antioxidants on biodiesel oxidation stability. Eur J Lipid Sci Technol 106(6):382–389
Ruger CW, Klinker EJ, Hammond EG (2002) Abilities of some antioxidants to stabilize soybean oil in industrial use conditions. J Am Oil Chem Soc 79(7):733–736
Farrington AM, Slater JM (1997) Monitoring of engine oil degradation by voltammetric methods utilizing disposable solid wire microelectrodes. Analyst 122(6):593
Clauss FJ (1972) Solid lubricants and self-lubricating solids. Academic Press, New York
Kanakia MD, Peterson MB, Southwest Research Institute, San Antonio, TX, Belvoir Fuels, Lubricants Research F (1987) Literature review of solid lubrication mechanisms. Defense Technical Information Center, Ft. Belvoir
Jamison WE (1972) Structure and bonding effects on the lubricating properties of crystalline solids. ASLE Trans 15(4):296–305
Winer W (1967) Molybdenum disulfide as a lubricant: a review of the fundamental knowledge. Wear 10(6):422–452
Wornyoh EYA, Jasti VK, Higgs CF (2007) A review of dry particulate lubrication: powder and granular materials. J Tribol 129(2):438–449
Peng Q, Ji W, De S (2012) Mechanical properties of the hexagonal boron nitride monolayer: ab initio study. Comput Mater Sci 56:11–17
Lelonis DA, Tereshko JW, Andersen CM (2007) Boron nitride powder: a high-performance alternative for solid lubrication. Momentive Performance Materials, Columbus
Clayton GD, Clayton FE, Allan RE, Patty FA (1991) Patty’s industrial hygiene and toxicology. Wiley, New York
Cermak SC, Isbell TA (2003) Improved oxidative stability of estolide esters. Ind Crops Prod 18(3):223–230
Findley TW, Swern D, Scanlan JT (1945) J Am Chem Soc 67(3):412–414
Rangarajan B, Havey A, Grulke EA, Dean Culnan P (1995) Kinetic parameters of a two-phase model for in situ epoxidation of soybean oil. J Am Oil Chem Soc 72(10):1161
Sonnet PE, Foglia TA (1996) Epoxidation of natural triglycerides with ethylmethyldioxirane. J Am Oil Chem Soc 73(4):461–464
Debal A, Rafaralahitsimba G, Ucciani E (1993) Epoxidation of fatty acid methyl esters with organic hydroperoxides and molybdenum oxide. Fat Sci Technol 95(6):236
Ucciani E, Bonfand A, Rafaralahitsimba G, Cecchi G (1992) Epoxidation of monoenic fatty esters with cumilhydroperoxide and hexacarbonyl-molybdenum. Revue Francaise Des Corps Gras 39(9/10):279
Debal A, Rafaralahitsimba G, Bonfand A, Ucciani E (1995) Catalytic epoxidation of methyl linoleate—cyclisation products of the epoxyacid esters. Fat Sci Technol 97(7/8):269
Semel J, Steiner R (1983) Renewable raw materials in the chemical industry. Nachr Chem Tech Lab 31(8):632–635
Klaas MRG, Warwel S (1996) Chemoenzymatic epoxidation of unsaturated fatty acid esters and plant oils. J Am Oil Chem Soc 73(11):1453
Kende AS, Mckusick BC (1987) Performic acid epoxidation. Chem Eng News 65(35):3–3
Klaas MRG, Warwel S (1999) Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind Crops Prod 9(2):125–132
Chou TC, Lee SV (1997) Epoxidation of oleic acid in the presence of benzaldehyde using cobalt(II) tetraphenylporphyrin as catalyst. Ind Eng Chem Res 36(5):1485–1490
Tocci L (2004) What does biodegradable really mean? Lubes ‘n’ Greases 10:40–43
Organisation for Economic, Cooperation and Development (2002) Guidance document for the development of OECD guidelines for testing of chemicals. Paris: OECD
Pagga U (1997) Testing biodegradability with standardized methods. Chemosphere 35(12):2953–2972
Willing A (1999) Oleochemical esters—environmentally compatible raw materials for oils and lubricants from renewable resources. Lipid 101(6):192–198
Remmele E, Widmann B (1998) Hydraulic fluids based on rapeseed oil in agricultural machinery-sustainability and environmental impact during use. In: Bartz WJ (ed) 11th international colloquium of industrial and automotive lubrication. TA Esslingen, Esslingen
Battersby N (1992) A correlation between the biodegradability of oil products in the CEC L-33-T-82 and modified Sturm tests. Chemosphere 24(12):1989–2000
Benchaita MT, Lockwood FE (1993) Reliable model of lubricant-related friction in internal combustion engines. Lubr Sci 5(4):259–281
Cermak SC, Isbell TA (2009) Synthesis and physical properties of mono-estolides with varying chain lengths. Ind Crops Prod 29(1):205–213
Johansson LE (1979) Copper catalysts in the selective hydrogenation of soybean and rapeseed oils. I. The activity of the copper chromite catalyst. J Am Oil Chem Soc 56:974–980
Behr A, Doring N, Durowitz-Heil S, Lohr C, Schmidtke H (1993) Selective hydrogenation of multi-unsaturated fatty-acids in the liquid-phase. Fat Sci Technol 95:2–11
Behr A (1990) Homogeneous transition-metal catalysis in oleochemistry. Fat Sci Technol 92:375–388
Fell B, Schafer W (1990) Selective hydrogenation of fats and derivatives using Ziegler-type organometallic catalysts. 1. Selective hydrogenation of methyllinoleat and other dienic compounds with isolated double-bonds. Fat Sci Technol 92:264–272
Haase KD, Heynen AJ, Laane NLM (1989) Composition and application of isostearic acid. Fat Sci Technol 91:350–353
Link W, Spitellar G (1990) Products of the dimerization of unsaturated fatty acids. I: the fraction of monomers obtained by dimerization of pure oleic acid. Fat Sci Technol 92:19–25
Perin G, Alvaro G, Westphal E, Jacob RG, Lenardao EJ, Viana LH, D’Oca MGM (2008) Transesterification of castor oil assisted by microwave irradiation. Fuel 87(12):2838–2841
Battersby NS, Morgan P (1997) A note on the use of the CEC L-33-A-93 test to predict the potential biodegradation of mineral oil based lubricants in soil. Chemosphere 35(8):1773–1779
Adhvaryu A, Biresaw G, Sharma BK, Erhan SZ (2006) Friction behavior of some seed oils: biobased lubricant applications. Ind Eng Chem Res 45(10):3735–3740
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM (2000) Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta 364(1–2):87–97
Adhvaryu A, Erhan SZ, Perez JM (2004) Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 257(3–4):359–367
Jahanmir S, Beltzer M (1986) An adsorption model for friction in boundary lubrication. ASLE Trans 29(3):423–430
Jahanmir S, Beltzer M (1986) Effect of additive molecular structure on friction coefficient and adsorption. J Tribol 108(1):109
Jahanmir S (1985) Chain length effects in boundary lubrication. Wear 102(4):331–349
Beltzer M, Jahanmir S (1987) Role of dispersion interactions between hydrocarbon chains in boundary lubrication. ASLE Trans 30(1):47–54
Beltzer M, Jahanmir S (1988) Effect of additive molecular structure on friction. Lubr Sci 1(1):3–26
Schey JA (1983) Tribology in metalworking: friction, lubrication, and wear. American Society for Metals, Metals Park
Liu X, Zhou F, Liang Y, Liu W (2006) Tribological performance of phosphonium based ionic liquids for an aluminum-on-steel system and opinions on lubrication mechanism. Wear 261(10):1174–1179
Reeves Carlton J, Menezes Pradeep L, Jen Tien-Chien, Lovell Michael R (2015) The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol Int 90:123–134
Sharma BK, Liu Z, Adhvaryu A, Erhan SZ (2008) One-pot synthesis of chemically modified vegetable oils. J Agric Food Chem 56(9):3049–3056
Hwang HS, Erhan SZ (2001) Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J Am Oil Chem Soc 78:1179–1184
Adamczewska JZ, Wilson D (1997) Development of ecologically responsive lubricants. J Synth Lubr 14(2):129–142
Coscione AR, Artz WE (2005) Vegetable oil stability at elevated temperatures in the presence of ferric stearate and ferrous octanoate. J Agric Food Chem 53(6):2088–2094
Dunn RO (2005) Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technol 86(10):1071–1085
Adhvaryu A, Sharma BK, Hwang HS, Erhan SZ, Perez JM (2006) Development of biobased synthetic fluids: application of molecular modeling to structure-physical property relationship. Ind Eng Chem Res 45(3):928–933
Fessenbecker A, Korff J (1995) Additive fuer oekologisch unbedenklichere Schmierstoffe. Tribol Schmierungstech 42(1):26
Roehrs I, Fessenbecker A (1997) A new additive for the hydrolytic and oxidative stabilization of ester based lubricants and greases. NLGI Spokesm 61(3):10–17
Boyde S (2000) Hydrolytic stability of synthetic ester lubricants. J Synth Lubr 16(4):297–312
Asadauskas S, Perez JM, Duda JL (1996) Oxidative stability and antiwear properties of high oleic vegetable oils. Lubr Eng Ill 52(12):877–882
Honary LAT (1996) An investigation of the use of soybean oil in hydraulic systems. Bioresour Technol 56(1):41–47
Lal K, Carrick V (1994) Performance testing of lubricants based on high oleic vegetable oils. J Synth Lubr 11(3):189
Ullmann F, Gerhartz W (1988) Ullmann’s encyclopedia of industrial chemistry. VCH, Winheim
Jayadas NH, Nair KP, Ajithkumar G (2005) Vegetable oils as base oil for industrial lubricants—evaluation oxidative and low temperature properties using TGA, DTA and DSC. In: World tribology congress. American Society of Mechanical Engineers, Washington, DC, pp 539–540
Quinchia LA, Delgado MA, Franco JM, Spikes HA, Gallegos C (2012) Low-temperature flow behaviour of vegetable oil-based lubricants. Ind Crops Prod 37(1):383–388
Hart H, Schuetz RD (1978) Organic chemistry: a short course. Houghton Mifflin, Boston
Miller S, Scharf C, Miller M (2002) Utilizing new crops to grow the biobased market. ASHS Press, Alexandria
Willing A (2001) Lubricants based on renewable resources-an environmentally compatible alternative to mineral oil products. Chemosphere 43(1):89–98
Torbacke TN, Kopp M (2002) Environmentally adapted lubricants in the Nordic marketplace—recent developments. Ind Lubr Tribol 54(3):109–116
USDA (2013) BioPreferred Program. http://www.biopreferred.gov/
Author information
Authors and Affiliations
Corresponding author
Terminologies and Nomenclature
Terminologies and Nomenclature
No. | Terminologies | Description |
|---|---|---|
1 | 1,3-Dialkylimidazolium cations (DAI) | A class of room temperature ionic liquids described as hydrogen-bonded polymeric supramolecules of the type {[(DAI) x (X) x−n ]n+, [(DAI) x−n (X) x )]n−} n , where X is the anion |
2 | 12-Hydroxystearic acid | A chemical compound classified as a lithium soap. In chemistry, “soap” refers to salts of fatty acids which are key components of lubricating greases |
3 | Acylation | The process of adding an acyl group to a compound |
4 | Acyloxylation | The process of substitution of a hydrogen atom by an acyloxy group |
5 | Additivation | Process treating oils and lubricants by adding additives to them |
6 | Adipic acid or maleic acid | A mixture of cyclohexanol and cyclohexanone called “KA oil,” the abbreviation of ketone-alcohol oil |
7 | Alkyl hydroperoxides | Organic compounds containing the peroxide functional group (ROOR′), with R being an alkyl group and R′ being the hydrogen |
8 | Alkylated aromatics | An alkylated hydrocarbon with sigma bonds and delocalized pi electrons between carbon atoms forming a circle |
9 | Alkylation | Alkylation is the transfer of an alkyl group from one molecule to another |
10 | Amino alkylation | Alkylated amines to form C–N bonds |
11 | Amphiphilic | A chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties |
12 | ASTM D2112-93, ASTM D2272, and ASTM D943 | Standard test methods for oxidation stability as described by ASTM |
13 | ASTM D2619 (beverage bottle test) and ASTM D943 (TOST test) | Standard test methods for examining hydrolytic stability as described by ASTM |
14 | ASTM standards D445 and D2270 | Standard Test Methods for Kinematic Viscosity and the viscosity index as described by ASTM |
15 | Azelaic acid | An organic compound with the formula consisting of saturated dicarboxylic acid existing as a white powder and found in wheat, rye, and barley |
16 | Baader Oxidation Test (DIN 51553 part 3) | A versatile test method for oxidation stability as described by VDMA (Verband Deutscher Maschinen- und Anlagenbau, Mechanical Engineering Industry Association) |
17 | Baader-test (DIN 51587) | A test method for oxidation stability as described by VDMA (Verband Deutscher Maschinen- und Anlagenbau, Mechanical Engineering Industry Association) |
18 | Bis-allylic hydrogen | They are two hydrogen atoms that are bonded to an allylic carbon in an organic molecule |
19 | Branched alcohols | Alcohol compounds which occur in isomeric structures, from a straight-chain primary alcohol to a branched-chain tertiary alcohol |
20 | Branched fatty acids | Saturated fatty acids with one or more methyl branches on the carbon chain |
21 | C5–C18 mono acids | Fatty acids that occur as their esters, commonly triglycerides, which are the greasy materials in many natural oils |
22 | C5–C9 carboxylic acids | Organic compounds that contain one or more carboxyl group. That include amino acids (which make up proteins) and acetic acid (which is part of vinegar and occurs in metabolism) |
23 | C6–C13 alcohols | These are aliphatic alcohol components, with a range (6–13) of carbon chain lengths |
24 | CECL-33-A-93 tests | Standard Test Methods of evaluating the infrared (IR) bands of the C–H bonds as described by CEC, Coordinating European Council |
25 | CECL-33-T-82 | Standard Test Methods of evaluating the minimum requirements of biodegradability as described by CEC, Coordinating European Council |
26 | Chemoenzymatic self-epoxidation process | A synthesis, which combines the flexibility of chemical synthesis and the high selectivity of enzymatic synthesis to obtain complex carbohydrates |
27 | Co-oligomerization | Oligomers derived from more than one species of monomer. An oligomer is one which consists of molecules of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass |
28 | DA-FPEs | α,ω-Dialkoxyfluoropolyethers |
29 | Diacids | Adipic acid, azelaic, sebacic, and dodecanedioic |
30 | EDTA | Ethyl-enediamine-tetra-acetic acid |
31 | Elaidic acid (trans C18:1) | An organic compound with 18 carbon fatty acid chains and one double bond of the fatty acid chain. It is an unsaturated fatty acid that is the most widely distributed and abundant fatty acid in nature. It is used commercially in the preparation of oleates and lotions and as a pharmaceutical solvent |
32 | Enereaction | Also known as the Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing multiple bonds (the enophile), in order to form a new σ-bond with the migration of the ene double bond and 1,5 hydrogen shift |
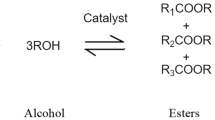
33 | Esterification or transesterification | The modification of the ester moieties present in the triacylglycerides, which have glycerol as the alcohol component and have the critical β-hydrogen, which is susceptible to thermal degradation by elimination |
34 | GMOs | Genetically modified organisms |
35 | HOSO | High-oleic acid safflower oil |
36 | Hydroformylation | Also known as oxo synthesis or oxo process is an industrial process for the production of aldehydes from alkenes |
37 | Hydrolytic degradation | Usually, hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both substance and water molecule to split into two parts. In such reactions, one fragment of the target molecule (or parent molecule) gains a hydrogen ion |
38 | Linoleic acid (C18:2) | A carboxylic acid with an 18-carbon chain and two cis double bonds, with the first double bond located at the sixth carbon from the methyl end |
39 | Linolenic acid (C18:3) | An organic compound with an 18-carbon chain and three cis double bonds classified as a keto acid. It is derived from degradation of cellulose and is a potential precursor to biofuels |
40 | Lipid modifiers | Organic compounds which are considered as lipid additives to modify specific properties of lipids |
41 | Lipid number | The numbers in the lipid name are used to describe the fatty acid chains on the lipid. The numbers are generally presented in the format (number of carbons in fatty acid chain):(number of double bonds in fatty acid chain) |
42 | Metathesis | A bimolecular process involving the exchange of bonds between the two reacting chemical species |
43 | Mono/di/tri/poly unsaturated fatty acids | Fatty acids that have one/two/three/poly double bond in the fatty acid chain with all of the remainder carbon atoms being single-bonded |
44 | Natural esters (type HETG) | Vegetable fluid-based hydraulic fluids |
45 | NPG | Neopentylglycol |
46 | Nucleophilic attacks | Nucleophilic substitution/attack is a fundamental class of reactions in which an electron-rich nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms to replace a leaving group; the positive or partially positive atom is referred to as an electrophile |
47 | OECD 201 through 213 tests and 401 | Standard method of testing the aquatic toxicity of lubricants by measuring the extent to which they poison particular environmental species as described by OECD, Organization of Economic Cooperation and Development |
48 | OECD 301 tests | Standard test methods for measuring total degradation that describes the conversion of the original organic compound to carbon dioxide (CO2) and water (H2O) by biodegradation as described by OECD, Organization of Economic Cooperation and Development |
49 | PAGs | Polyalkylene glycols |
50 | PAOs | Polyalphaolefins |
51 | PE | Pentaerythritol |
52 | PFPEs | Perfluoroalkylethers |
53 | PGs | Polyglycols |
54 | PTSA | p-Toluenesulfonic acid |
55 | Polyglycols (type HEPG) | Polyglycol-based synthetic hydraulic fluids |
56 | Rancimat method | Method to determine the oxidation stability of natural fats and oils, in their pure form as well as in fat-containing foods and cosmetics |
57 | Selective hydrogenation | The process of converting polyunsaturated fatty acids to saturated and monounsaturated fatty acids by removing all or all but one of the double bonds |
58 | Synthetic esters (type HEES) | Ester-based synthetic hydraulic fluids |
59 | TGA | Thermogravimetric analysis |
60 | TME | Trimethylolethane |
61 | TMH | Trimethylolhexane |
62 | TMP | Trimethylolpropane |
63 | Transesterification | The process of exchanging the organic group of an ester with the organic group of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst |
64 | Two One-Sided Tests (DIN 51587)/TOST test | Requirements of the thermal-oxidation stability test (TOST) in accordance with DIN 51587 (German Institute of Standardization) |
65 | USDA | The United States Department of Agriculture |
Rights and permissions
About this article
Cite this article
Reeves, C.J., Siddaiah, A. & Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J Bio Tribo Corros 3, 11 (2017). https://doi.org/10.1007/s40735-016-0069-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-016-0069-5