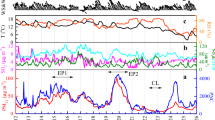
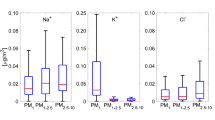
Abstract
Atmospheric new particle formation and growth play important roles in climate change and air quality. Aiming at better understanding the particle growth mechanisms, the measurements on chemical composition of new particles are imperative. However, the instruments directly detecting chemical composition of nanoparticles (<30 nm) are very rare due to the tiny particle masses involved and low transmission efficiency. Alternatively, the hygroscopicity and volatility of nanoparticles were measured to infer chemical composition of the particle. Here, we summarized the progresses in studying the new particle growth processes on a basis of particle hygroscopicity and volatility measurements. Compared to clean environments, such as in boreal forest, the water soluble components contribute a larger fraction of newly formed particles (below 50 nm) in the polluted environments, such as in the sulfur-rich atmosphere of North China Plain. The extreme low volatility components in new particles were observed in both clean and polluted environments and contributed to 1/4 of particle growth in a rural site of Melpitz, Germany. In the future, the instruments capable of precisely detecting the hygroscopicity and volatility of particles below 10 nm are needed. Except for differential mobility analyzer, other novel methods without limitation of charging and transmission efficiency should be considered. The hygroscopicity and volatility of atmospheric relevant compounds should be investigated in the laboratory in order to provide supportive information to explain the hygroscopicity and volatility of new particle in the ambient air.
Similar content being viewed by others
Reference
Kulmala M, Vehkamäki H, Petäjä T, Dal Maso M, Lauri A, Kerminen VM, et al. Formation and growth rates of ultrafine atmospheric particles: a review of observations. J Aerosol Sci. 2004;35(2):143–76.
Kulmala M, Arola A, Nieminen T, Riuttanen L, Sogacheva L, de Leeuw G, et al. The first estimates of global nucleation mode aerosol concentrations based on satellite measurements. Atmos Chem Phys. 2011;11(21):10791–801.
Spracklen DV, Carslaw KS, Kulmala M, Kerminen V-M, Sihto S-L, Riipinen I, et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophys Res Lett. 2008;35(6):L06808.
Wiedensohler A, Cheng YF, Nowak A, Wehner B, Achtert P, Berghof M, et al. Rapid aerosol particle growth and increase of cloud condensation nucleus activity by secondary aerosol formation and condensation: A case study for regional air pollution in northeastern China. J Geophys Res. 2009;114(D2):D00G08.
Wang M, Penner JE. Aerosol indirect forcing in a global model with particle nucleation. Atmos Chem Phys. 2009;9(1):239–60.
Laaksonen A, Hamed A, Joutsensaari J, Hiltunen L, Cavalli F, Junkermann W, et al. Cloud condensation nucleus production from nucleation events at a highly polluted region. Geophys Res Lett. 2005;32(6):L06812.
Yue DL, Hu M, Zhang RY, Wu ZJ, Su H, Wang ZB, et al. Potential contribution of new particle formation to cloud condensation nuclei in Beijing. Atmos Environment. 2011;45(33):6070–7.
Kazil J, Stier P, Zhang K, Quaas J, Kinne S, O’Donnell D, et al. Aerosol nucleation and its role for clouds and Earth’s radiative forcing in the aerosol-climate model ECHAM5-HAM. Atmos Chem Phys. 2010;10:10733–52. doi.10.5194/acp-10-10733-2010. Accessed 15 July 2017.
Guo S, Hu M, Zamora ML, Peng J, Shang D, Zheng J, et al. Elucidating severe urban haze formation in China. Proc Natl Acad Sci USA. 2014;111(49):17373–8.
Wiedensohler A, Cheng YF, Nowak A, Wehner B, Achtert P, Berghof M, et al. Rapid aerosol particle growth and increase of cloud condensation nucleus activity by secondary aerosol formation and condensation: A case study for regional air pollution in northeastern China. J Geophys Res Atmos. 2009;114(D2):D00G08.
Xiao S, Wang MY, Yao L, Kulmala M, Zhou B, Yang X, et al. Strong atmospheric new particle formation in winter in urban Shanghai, China. Atmos Chem Phys. 2015;15(4):1769–81.
An J, Wang H, Shen L, Zhu B, Zou J, Gao J, et al. Characteristics of new particle formation events in Nanjing, China: effect of water-soluble ions. Atmos Environ. 2015;108:32–40.
Qi XM, Ding AJ, Nie W, Petäjä T, Kerminen VM, Herrmann E, et al. Aerosol size distribution and new particle formation in the western Yangtze River Delta of China: 2 years of measurements at the SORPES station. Atmos Chem Phys. 2015;15(21):12445–64.
Smith JN, Moore KF, McMurry PH, Eisele FL. Atmospheric measurements of sub-20 nm diameter particle chemical composition by thermal desorption chemical ionization mass spectrometry. Aerosol Sci Technol. 2004;38(2):100–10.
Smith JN, Barsanti KC, Friedli HR, Ehn M, Kulmala M, Collins DR, et al. Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc Natl Acad Sci U S A. 2010;107:6634–9.
Wang S, Johnston MV. Airborne nanoparticle characterization with a digital ion trap–reflectron time of flight mass spectrometer. Int J Mass Spectrom. 2006;258(1–3):50–7.
Väkevä M, Kulmala M, Stratmann F, Hämeri K. Field measurements of hygroscopic properties and state of mixing of nucleation mode particles. Atmos Chem Phys. 2002;2(1):55–66.
Sakurai H, Fink MA, McMurry PH, Mauldin L, Moore KF, Smith JN, et al. Hygroscopicity and volatility of 4–10 nm particles during summertime atmospheric nucleation events in urban Atlanta. J Geophys Res. 2005;110(D22):D22S04.
Wehner B, Petäjä T, Boy M, Engler C, Birmili W, Tuch T, et al. The contribution of sulfuric acid and non-volatile compounds on the growth of freshly formed atmospheric aerosols. Geophys Res Lett. 2005;32(17):L17810.
Ehn M, Petäjä T, Aufmhoff H, Aalto P, Hämeri K, Arnold F, et al. Hygroscopic properties of ultrafine aerosol particles in the boreal forest: diurnal variation, solubility and the influence of sulfuric acid. Atmos Chem Phys. 2007;7(1):211–22.
Petäjä T, Kerminen VM, Dal Maso M, Junninen H, Koponen IK, Hussein T, et al. Sub-micron atmospheric aerosols in the surroundings of Marseille and Athens: physical characterization and new particle formation. Atmos Chem Phys. 2007;7(10):2705–20.
Asmi E, Frey A, Virkkula A, Ehn M, Manninen HE, Timonen H, et al. Hygroscopicity and chemical composition of Antarctic sub-micrometre aerosol particles and observations of new particle formation. Atmos Chem Phys. 2010;10(9):4253–71.
Ristovski ZD, Suni T, Kulmala M, Boy M, Meyer NK, Duplissy J, et al. The role of sulphates and organic vapours in growth of newly formed particles in a eucalypt forest. Atmos Chem Phys. 2010;10(6):2919–26.
Modini RL, Ristovski ZD, Johnson GR, He C, Surawski N, Morawska L, et al. New particle formation and growth at a remote, sub-tropical coastal location. Atmos Chem Phys. 2009;9(19):7607–21.
Virkkula A, Van Dingenen R, Raes F, Hjorth J. Hygroscopic properties of aerosol formed by oxidation of limonene, α-pinene, and β-pinene. J Geophys Res. 1999;104(D3):3569–79.
Varutbangkul V, Brechtel FJ, Bahreini R, Ng NL, Keywood MD, Kroll JH, et al. Hygroscopicity of secondary organic aerosols formed by oxidation of cycloalkenes, monoterpenes, sesquiterpenes, and related compounds. Atmos Chem Phys. 2006;6(9):2367–88.
Tang IN, Munkelwitz HR. Water activities, densities, and refractive indices of aqueous sulfates and sodium nitrate droplets of atmospheric importance. J Geophys Res. 1994;99(D9):18801–8.
Hämeri K, Väkevä M, Aalto PP, Kulmala M, Swietlicki E, Zhou J, et al. Hygroscopic and CCN properties of aerosol particles in boreal forests. Tellus B. 2001;53(4):359–79.
Cappa CD, Wilson KR. Evolution of organic aerosol mass spectra upon heating: implications for OA phase and partitioning behavior. Atmos Chem Phys. 2011;11(5):1895–911.
Wu ZJ, Nowak A, Poulain L, Herrmann H, Wiedensohler A. Hygroscopic behavior of atmospherically relevant water-soluble carboxylic salts and their influence on the water uptake of ammonium sulfate. Atmos Chem Phys. 2011;11(24):12617–26.
Massling A, Wiedensohler A, Busch B, Neusüß C, Quinn P, Bates T, et al. Hygroscopic properties of different aerosol types over the Atlantic and Indian Oceans. Atmos Chem Phys. 2003;3(5):1377–97.
Zdanovskii B. Novyi Metod Rascheta Rastvorimostei Elektrolitov v Mnogokomponentnykh Sistema. ZhFiz Khim+. 1948;22(12):1478–85. 1486-1495
Stokes RH, Robinson RA. Interactions in aqueous nonelectrolyte solutions. I Solute-Solvent Equilibria. J Phys Chem. 1966;70:2126–30.
Malm WC, Kreidenweis SM. The effects of models of aerosol hygroscopicity on the apportionment of extinction. Atmos Environment. 1997;31(13):1965–76.
Swietlicki E, Zhou J, Berg OH, Martinsson BG, Frank G, Cederfelt S-I, et al. A closure study of sub-micrometer aerosol particle hygroscopic behaviour. Atmos Res. 1999;50(3–4):205–40.
Potukuchi S, Wexler AS. Identifying solid-aqueous phase transitions in atmospheric aerosols—I. Neutral-acidity solutions. Atmos Environment. 1995;29(14):1663–76.
Wehner, B.; Petäjä, T.; Boy, M.; Engler, C.; Birmili, W.; Tuch, T.; Wiedensohler, A.; Kulmala, M., The contribution of sulfuric acid and non-volatile compounds on the growth of freshly formed atmospheric aerosols. Geophys Res lett 2005;32(17).
Philippin S, Wiedensohler A, Stratmann F. Measurements of non-volatile fractions of pollution aerosols with an eight-tube volatility tandem differential mobility analyzer (VTDMA-8). J Aerosol Sci. 2004;35(2):185–203.
Johnson GR, Ristovski Z, Morawska L. Method for measuring the hygroscopic behaviour of lower volatility fractions in an internally mixed aerosol. J Aerosol Sci. 2004;35(4):443–55.
Laaksonen A, Kulmala M, O'Dowd CD, Joutsensaari J, Vaattovaara P, Mikkonen S, et al. The role of VOC oxidation products in continental new particle formation. Atmos Chem Phys. 2008;8(10):2657–65.
Wang Z, Su H, Wang X, Ma N, Wiedensohler A, Pöschl U, et al. Scanning supersaturation condensation particle counter applied as a nano-CCN counter for size-resolved analysis of the hygroscopicity and chemical composition of nanoparticles. Atmos Meas Tech. 2015;8(5):2161–72.
Dusek, U.; Frank, G. P.; Curtius, J.; Drewnick, F.; Schneider, J.; Kürten, A.; Rose, D.; Andreae, M. O.; Borrmann, S.; Pöschl, U., Enhanced organic mass fraction and decreased hygroscopicity of cloud condensation nuclei (CCN) during new particle formation events. Geophys Res Lett. 2010;37(3):L03804.
Wu ZJ, Poulain L, Birmili W, Größ J, Niedermeier N, Wang ZB, et al. Some insights into the condensing vapors driving new particle growth to CCN sizes on the basis of hygroscopicity measurements. Atmos Chem Phys. 2015;15(22):13071–83.
Wu ZJ, Ma N, Größ J, Kecorius S, Lu KD, Shang DJ, et al. Thermodynamic properties of nanoparticles during new particle formation events in the atmosphere of North China Plain. Atmos Res. 2017;188:55–63.
Sakurai H, Fink MA, McMurry PH, Mauldin L, Moore KF, Smith JN, et al. Hygroscopicity and volatility of 4–10 nm particles during summertime atmospheric nucleation events in urban Atlanta. J Geophys Res Atmos. 2005;110(D22):3033–43.
Jung J, Kawamura K. Hygroscopic properties of newly formed ultrafine particles at an urban site surrounded by deciduous forest (Sapporo, northern Japan) during the summer of 2011. Atmos Chem Phys. 2014;14(14):7519–31.
Väkevä M, Hämeri K, Aalto PP. Hygroscopic properties of nucleation mode and Aitken mode particles during nucleation bursts and in background air on the west coast of Ireland. J Geophys Res Atmos. 2002;107(D19):PAR 9–1–PAR 9–11.
Stolzenburg MR, McMurry PH, Sakurai H, Smith JN, Mauldin RL, Eisele FL, et al. Growth rates of freshly nucleated atmospheric particles in Atlanta. J. Geophys. Res. Atmos. 2005;110(D22):D22S05.
Yue DL, Hu M, Zhang RY, Wang ZB, Zheng J, Wu ZJ, et al. The roles of sulfuric acid in new particle formation and growth in the mega-city of Beijing. Atmos Chem Phys. 2010;10(10):4953–60.
Vakkari V, Tiitta P, Jaars K, Croteau P, Beukes JP, Josipovic M, et al. Reevaluating the contribution of sulfuric acid and the origin of organic compounds in atmospheric nanoparticle growth. Geophys Res Lett. 2015;42(23):10,486–93.
Ehn M, Petäjä T, Birmili W, Junninen H, Aalto P, Kulmala M. Non-volatile residuals of newly formed atmospheric particles in the boreal forest. Atmos Chem Phys. 2007;7(3):677–84.
Wang, Z.; Birmili, W.; Hamed, A.; Wehner, B.; Spindler, G.; Pei, X.; Wu, Z.; Cheng, Y.; Su, H.; Wiedensohler, A., Contributions of volatile and non-volatile compounds (at 300 °C) to condensational growth of atmospheric nanoparticles: an assessment based on 8.5 years of observations at the Central Europe background site Melpitz. J Geophys Res-Atmos.2016.
Wang Z, Birmili W, Hamed A, Wehner B, Spindler G, Pei X, et al. Contributions of volatile and nonvolatile compounds (at 300°C) to condensational growth of atmospheric nanoparticles: an assessment based on 8.5 years of observations at the Central Europe background site Melpitz. J. Geophys. Res Atmos. 2017;122(1):485–97.
Huffman JA, Ziemann PJ, Jayne JT, Worsnop DR, Jimenez JL. Development and characterization of a fast-stepping/scanning thermodenuder for chemically-resolved aerosol volatility measurements. Aerosol Sci Technol. 2008;42(5):395–407.
Lee B-H, Pierce JR, Engelhart GJ, Pandis SN. Volatility of secondary organic aerosol from the ozonolysis of monoterpenes. Atmos Environment. 2011;45(14):2443–52.
Kalberer M, Paulsen D, Sax M, Steinbacher M, Dommen J, Prevot ASH, et al. Identification of polymers as major components of atmospheric organic aerosols. Science. 2004;303(5664):1659–62.
Wang L, Khalizov AF, Zheng J, Xu W, Ma Y, Lal V, et al. Atmospheric nanoparticles formed from heterogeneous reactions of organics. Nat Geosci. 2010;3(4):238–42.
Acknowledgements
This work is supported by the following projects: National Natural Science Foundation of China (41475127, 41571130021). The authors would like to greatly thank Zhibin Wang for useful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Air Pollution
Rights and permissions
About this article
Cite this article
Wu, Z. Gain Insight into Chemical Components Driving New Particle Growth on a Basis of Particle Hygroscopicity and Volatility Measurements: a Short Review. Curr Pollution Rep 3, 175–181 (2017). https://doi.org/10.1007/s40726-017-0064-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-017-0064-6