Abstract
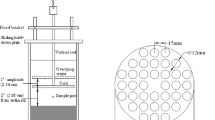
Cadmium desorption from the river bed sediments has been experimentally investigated. Artificially contaminated sediments with cadmium (Cd) were prepared for performing batch desorption experiments. The experiments were conducted by adding 1 g of contaminated sediment (D50 = 0.53 mm), containing different amounts of adsorbed Cd, to 50 mL of distilled water at different times (0, 5, 15, 30, 60, 120, 300, and 720 min) and shaking to reach an equilibrium desorption rate. In addition, the experiments were conducted for two agitation rates of 100 and 200 rpm. It was concluded that the cadmium ions were strongly bond to the river bed sediment; meanwhile, at the equilibrium time, up to about 7 to 29% of cadmium ions were released from the artificially contaminated sediments. It was also revealed that by increasing the flow turbulence, the amount of desorbed cadmium is slightly increased. Besides, the desorption kinetics was evaluated using eight models: zero-, first-, second-, third-order, parabolic diffusion, double parabolic diffusion, two-constant rate, and simple Elovich. The results of the evaluation showed that simple Elovich (with R2 = 0.991), double parabolic diffusion (with R2 = 0.9882), two constant rate (with R2 = 0.983), and parabolic diffusion models (with R2 = 0.846) have the best performance in calculation of Cd desorption rate from the sediments. The results of this study on kinetics of Cd adsorption and desorption onto/from the natural sediments may be useful for predicting efficiency of heavy metal decontamination in rivers.











Similar content being viewed by others
Data Availability
Some or all data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmaruzzaman M (2011) Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv Colloid Interface Sci 166:36–59
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332
Allen HE, Chen YT, Li YM, Huang CP (1995) Soil partition coefficients for Cadmium by column desorption and comparison to batch adsorption measurements. Envir Sci Technol 29(8):1887–1891
Amela K, Hassen MA, Kerroum D (2012) Isotherm and kinetics study of biosorption of cationic dye onto banana Peel. Energy Procedia 19:286–295
Atkinson RJ, Hingston FJ, Posner AM, Quirk JP (1970) Elovich equation for the kinetics of isotope exchange reactions at solid-liquid interfaces. Nature (London) 226:148–149
Bower CA, Reitmeir RF, Fireman M (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73:251–261
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Cheng H, Hu E, Hu Y (2012) Impact of mineral micropores on transport and fate of organic contaminants: A review. J Contam Hydrol 129–130:80–90
Cheng QM, Huang Q, Khan S, Liu YJ, Liao ZN, Li G, Ok YS (2016) Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecological Engineering 87:240–245. https://doi.org/10.1016/j.ecoleng.2015.11.045
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci Soc Am J 44:265–268
Dang YP, Dalal DG, Edwards DG, Tiller KG (1994) Kinetics of zink adsorption from vertisols. Soil Sci America J 58:1392–1399
Esfandbod M, Adhami E, Rezaei Rashti CM, Esfandbod M (2010) Kinetics of cadmium desorption from some soils of Iran. 19th World Congress of Soil Science, Soil Solutions for a Changing World. 1–6 August, Brisbane, Australia, 154-157
Fornster U, Gottfried TW (1981) Metal pollution in the aquatic environment, 2nd edn. Springer-Verlag., Berlin
Franchi A, Davis AP (1997) Desorption of cadmium (ii) from artificially contaminated sediments. Water Air Soil Pollut 100:181–196
Fu L, Xu X, Fu G, Zhang R, Liu H (2020) Adsorption and desorption characteristics of cadmium ion by ash-free biochars. J Renew Mater 8(7):801–818. https://doi.org/10.32604/jrm.2020.09369
Gao Y, Kan AT, Tomson MB (2003) Critical evaluation of desorption phenomena of heavy metals from natural sediments. Environ Sci Technol 37(24):5566–5573. https://doi.org/10.1021/es034392w
Gardiner J (1974) The chemistry of cadmium in natural waters. The adsorption of cadmium in river muds and naturally occurring soils. Water Res 8:157–164
Ghasemi Fasaei R, Maftoun M, Ronaghi A, Karimian N, Yasrebi J, Assad MT, Ippolito JA (2006) Kinetics of copper desorption from highly calcareous soils. Commun Soil Sci Plant Analysis 37:797–809
Ghoveisi H, Mahdavi Mazdeh A, Farhoudi J, Omid MH (2014) The effect of sediment motion on adsorption of cadmium. Proc ICE - Water Manag 167(4):238–245. https://doi.org/10.1680/wama.12.00103
González Costa JJ, Reigosa MJ, Matías JM, Covelo EF (2017) Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: variable selection and competitive model. Sci Total Environ 508:593–594. https://doi.org/10.1016/j.scitotenv.2017.03.195
Hart BT (1986) Water Quality Management-The role of particulate matter in the transport and fate of pollutants. Water Studies Center, Chisholm Institute of Technology, Melbourne
Hingston FJ (1981) A review of anion adsorption. In: Anderson MA, Rubin AJ (eds) Adsorption of Inorganics at Solid-Liquid Surfaces. Ann Arbor Science, Ann Arbor, pp 51–90
Huang SL (2003) Investigation of cadmium desorption from different sized sediments. J Environ Eng 129(3):–241
Huang SL, Wan ZH (1995) Present state of experimental research on heavy metal pollutant adsorption-desorption by sediment. Int J Sedim Res 10(3):69–81
Huang SL, Onyx Wai WH, Sheung Li Y (1999) Determination of cadmium ion desorption from non-uniform sediment particles. Toxicol Environ Chem 72(3–4):195–213
Huang Y, Fu C, Li Z, Fang F, Ouyang W, Guo J (2019) Effect of dissolved organic matters on adsorption and desorption behavior of heavy metals in a water-level-fluctuation zone of the Three Gorges Reservoir, China. Ecotoxicol Environ Saf 185:109695. https://doi.org/10.1016/j.ecoenv.2019.109695
Ilieva D, Surleva AR, Murariu M (2018) Evaluation of ICP-OES method for heavy metal and metalloids determination in sterile dump material. Bull Karaganda Univ 723(3):159–166. https://doi.org/10.4028/www.scientific.net/SSP.273.159
Jain CK, Ram D (1997a) Adsorption of lead and zinc on bed sediments of the river Kali. Water Res 31(1):154–162
Jain CK, Ram D (1997b) Adsorption of metal ions on bed sediments. Hydrol Sci J 42(5):713–723
Jain CK, Sharma MK (2002) Adsorption of cadmium on bed sediment of river Hindon: adsorption models and kinetics. Water Air Soil Pollut 137:1–19
Kaakani MW (2012) Heavy Metal removal from Wastewater using novel Adsorbent, Master’s Thesis, American University of Sharjah
Kazemi A, Esmaeilbeigi M, Sahebi Z, Ansari A (2022) Health risk assessment of total chromium in the qanat as historical drinking water supplying system. Sci Total Environ 807:150795. https://doi.org/10.1016/j.scitotenv.2021.150795
Khater AH, Zaghloul AM (2002) Copper and zink desorption kinetics from soil: Effect of pH, paper presented in 17th World Conference on Soil science, 14-21 August, Thailand, 47: 1-9
Krishnamurti GSR, Cieslinski G, Huang PM, Van Rees KCJ (1999) Kinetics of cadmium release from soils as influenced by organic acids: Implication in cadmium availability. J Environ Qual 26:271–277
Li P, Lang M, Wang XX, Zhang TL (2016) Sorption and desorption of copper and cadmium in a contaminated soil affected by soil amendments. CLEAN-Soil, Air 44(11):1547–1556. https://doi.org/10.1002/clen.201500555
Li Z, Man N, Wang S, Liang D (2015) Selenite adsorption and desorption in main Chinese soils with their characteristics and physicochemical properties. J Soils Sediments 15(5):1150–1158
Liu J, Liu YJ, Liu Y, Liu Z, Ning Zhang A (2018) Quantitative contributions of the major sources of heavy metals in soils to ecosystem and human health risks: A case study of Yulin, China. Ecotoxicol Environ Saf 164:261–269. https://doi.org/10.1016/j.ecoenv.2018.08.030
Loganathan P, Vigneswaran S, Kandasamy J, Naidu R (2012) Cadmium sorption and desorption in soils: a review. Crit Rev Environ Sci Technol 42(5):489–533. https://doi.org/10.1080/10643389.2010.520234
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 6:267–312
Lukman S, Essa MH, Muazu ND, Bukhari A, Basheer C (2013) Adsorption and desorption of heavy metals onto natural clay material: influence of initial pH. J Environ Sci Technol 6(1):1–15. https://doi.org/10.3923/jest.2013.1.15
Mahdavi A, Kashefipour SM, Omid MH (2013) Effect of sorption process on cadmium transport. Proc Inst Civ Eng Water Manag 166(3):152-162
Mahdavi A, Omid MH, Ganjali MR (2008) Effect of bed load transport on kinetic sorption in a circular flume. Proceedings of International Conference on Fluvial Hydraulics, RiverFlow2008, Cesme-Izmir, Turkey. Kubaba Congress Department and Travel Services, 3-5 September, Ankara, Turkey, 2485–2491
Markiewicz-Patkowska J, Hursthouse A, Przybyla-Kij H (2005) The interaction of heavy metals with urban soils: sorption behaviour of Cd, Cu, Cr, Pb and Zn with a typical mixed brownfield deposit. Environ Int 31:513
Mohammadi A, Saeedi M, Mollahoseini A (2018) Simultaneous desorption and desorption kinetics of phenanthrene, anthracene, and heavy metals from kaolinite with different organic matter content. Soil Sediment Contam 27(3):200–220. https://doi.org/10.1080/15320383.2017.1339666
Nasrabadi M, Omid MH, Mahdavi Mazdeh A (2017) Cadmium adsorption characteristics for Karaj Riverbed Sands. J Mate Environ Sci 8(5):1729–1736
Nasrabadi M, Omid MH, Mazdeh AM, Shahriari T (2018) Cadmium adsorption by natural zeolite in a circular flume. Int J Environ Technol Manag 21(3–4):174–189
Nasrabadi M, Omid MH, Mahdavi Mazdeh A (2021) Effect of cadmium sorption by river sediments on longitudinal dispersion. Water Sci Technol-Water Supply. https://doi.org/10.2166/ws.2021.368
Polyzopoulos NA, Keramidas VZ, Kiosse H (1986) Phosphate sorption by some alfisols of Greece as Described by Commonly Used isotherms. J Soil Sci 37:183–189. https://doi.org/10.2136/sssaj1985.03615995004900010016x
Putro JN, Santoso SP, Ismadji S, Ju YH (2017) Investigation of heavy metal adsorption in binary system by nanocrystalline cellulose—Bentonite nanocomposite: Improvement on extended Langmuir isotherm model. Microporous Mesoporous Mater 246:166–177
Reyhanitabar A, Gilkes RJ (2010) Kinetics of DTPA extraction of zinc from calcareous soils from Iran. 19th World Congress of Soil Science, Soil Solutions for a Changing World. 1–6 August, Brisbane, Australia, 19-22
Reyhanitabar A, Karimian N (2008) Kinetics of copper desorption of selected calcareous soils from Iran American-Eurasian. J Agric Environ Sci 4(3):287–293
Ushakumary ER (2013) Waste water treatment using low cost natural adsorbents, PhD Thesis, Cochin University of Science and Technology, India
Vibhawari B, Pandey ND (2010) Single and competitive sorption of heavy metal ions (Cd2+ & Cu2+) on a clayey soil. J Chem 7:S27–S34. https://doi.org/10.1155/2010/710546
Yongkui Y, Li L, Dingyong W (2008) Effect of dissolved organic matter on adsorption and desorption of mercury by soils. J Environ Sci 20:1097–1102
Author information
Authors and Affiliations
Contributions
Mohsen Nasrabadi, as former PhD Candidate, performed the experiments and analyzed and interpreted the data and he had a major contributor in writing the manuscript. Mohammad H. Omid is the main supervisor of the PhD thesis and Ali Mahdavi Mazdeh is the co-supervisor of the PhD thesis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasrabadi, M., Omid, M.H. & Mazdeh, A.M. Experimental Study of Flow Turbulence Effect on Cadmium Desorption Kinetics from Riverbed Sands. Environ. Process. 9, 10 (2022). https://doi.org/10.1007/s40710-022-00558-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-022-00558-y