Abstract
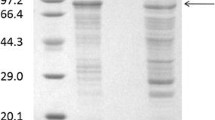
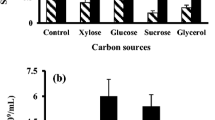
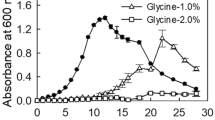
Bacillus subtilis UPMB13 was found to be an L-glutamic acid independent producer of extracellular polymeric substances (EPS) with bioflocculation properties. Optimum production of the bioflocculant was found to be at the early stage of cell propagation of 24–72 h of fermentation. At a limited nutrient input of 100 mL tryptic soy broth, the flocculating activities were found to be negatively correlated (p < 0.01) with growth as it continued to decline after 72 h, while cell growth proliferated further. Ample nutrient supply may prolong bioflocculant production with flocculating activities of 90 % and higher, while excess oxygen supply may promote rapid growth that can lead to poor flocculation due to the re-use of the bioflocculant as a substitute for food during starvation. Bioflocculant production occurred at best at 25-30 °C incubation temperature and at the initial pH medium of 7 to 8. The bioflocculant was proven to be extracellularly produced as the broth and the supernatant possessed the ability to flocculate the suspended kaolin particles. Bioflocculant productions by UPMB13 were hereditarily stable among succeeding progenies, hence, proving genetic competency. About 0.90 g of purified bioflocculant were collected from 1 L culture broth of UPMB13 under the optimized fermentation conditions.








Similar content being viewed by others
References
Aljuboori AHR, Idris A, Abdullah N, Mohamad R (2013) Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresource Technol 127:489–493. doi:10.1016/j.biortech.2012.09.016
Amir HG, Shamsuddin ZH, Halimi MS, Ramlan MF, Marziah M (2003) N2 fixation, nutrient accumulation and plant growth promotion by rhizobacteria in association with oil palm seedlings. Pakistan J Biol Sci 6:1269–1272
Bajaj I, Singhal R (2011a) Poly (glutamic acid): an emerging biopolymer of commercial interest. Bioresource Technol 102:5551–5561. doi:10.1016/j.biortech.2011.02.047
Bajaj I, Singhal R (2011b) Flocculation properties of poly(γ-glutamic acid) produced from Bacillus subtilis isolate. Food Bioprocess Tech 4:745–752. doi:10.1007/s11947-009-0186-y
Bhunia B, Mukhopadhy D, Goswami S, Mandal T, Dey A (2012) Improved production, characterization and flocculation properties of poly (γ)-glutamic acid produced from Bacillus subtilis. J Biochem Technol 3:389–394
Chen X, Chen S, Sun M, Yu Z (2005) Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl Microbiol Biot 69:390–396. doi:10.1007/s00253-005-1989-z
Cosa S, Ugbenyen AM, Mabinya LV, Rumbold K, Okoh AI (2013) Characterization and flocculation efficiency of a bioflocculant produced by a marine Halobacillus. Environ Technol 34:2671–2679. doi:10.1080/09593330.2013.786104
Devesa-Rey R, Bustos G, Cruz J, Moldes A (2012) Evaluation of non-conventional coagulants to remove turbidity from water. Water Air Soil Poll 223:591–598. doi:10.1007/s11270-011-0884-8
Dierksen KP, Sandine WE, Trempy JE (1997) Expression of ropy and mucoid phenotypes in Lactococcus lactis. J Dairy Sci 80:1528–1536. doi:10.3168/jds.S0022-0302(97)76082-X
Ito Y, Tanaka T, Ohmachi T, Asada Y (1996) Glutamic acid independent production of poly (y-glutamic acid) by Bacillus subtilis TAM-4. Biosci Biotech Bioch 60:1239–1242. doi:10.1271/bbb.60.1239
Kimura K, Tran LSP, Uchida I, Itoh Y (2004) Characterization of Bacillus subtilis gamma-glutamyltransferase and its involvement in the degradation of capsule poly-gamma-glutamate. Microbiol 150:4115–4123. doi:10.1099/mic.0.27467-0
Korsten L, Cook N (1996) Optimizing culturing conditions for Bacillus subtilis. South Afric Avocado Grow Assoc Yearbook 19:54–58
Liu LF, Cheng W (2010) Characteristics and culture conditions of a bioflocculant produced by Penicillium sp. Biomed Environ Sci 23:213–218. doi:10.1016/S0895-3988(10)60055-4
Mahmoud DAR (2006) Isolation of polyglutamic acid flocculant producing bacteria from extreme egyptian environments. Journal of Applied Science Research 2:608–612
Maximova N, Dahl O (2006) Environmental implications of aggregation phenomena: current understanding. Curr Opin Colloid In 11:246–266. doi:10.1016/j.cocis.2006.06.001
Muthulakshmi L, Nellaiah H, Busi S (2013) Production and characterization of a novel bioflocculant from Klebsiella sp. Curr Biot 2:53–58
Ntsaluba L, Nwodo UU, Mabinya L, Okoh A (2013) Studies on bioflocculant production by a mixed culture of Methylobacterium sp. obi and Actinobacterium sp. mayor. BMC Biotechnol 13:1–7. doi:10.1186/1472-6750-13-62
Nwodo UU, Green E, Mabinya LV, Okaiyeto K, Rumbold K, Obi LC, Okoh AI (2014) Bioflocculant production by a consortium of Streptomyces and Cellulomonas species and media optimization via surface response model. Colloid Surface B 116:257–264. doi:10.1016/j.colsurfb.2014.01.008
Nwodo UU, Okoh AI (2012) Characterization and flocculation properties of biopolymeric flocculant (glycosaminoglycan) produced by Cellulomonas sp. Okoh J Appl Microbiol 114:1325–1337. doi:10.1111/jam.12095
Okaiyeto K, Nwodo UU, Mabinya LV, Okoli AS, Okoh AI (2015) Characterization of a bioflocculant (MBF-UFH) produced by Bacillus sp. AEMREG7. Int J Mol Sci 16:12986–13003. doi:10.3390/ijms160612986
Patil S, Salunkhe R, Patil C, Patil D, Salunke B (2010) Bioflocculant exopolysaccharide production by Azotobacter indicus using flower extract of Madhuca latifolia L. Appl Biochem Biotech 162:1095–1108. doi:10.1007/s12010-009-8820-8
Patil SV, Bathe GA, Patil AV, Patil RH, Salunkea BK (2009) Production of bioflocculant exopolysaccharide by Bacillus subtilis. Adv Biot 8:14–17
Peirui L, Zongwei L, Zongyi L, Guangyong Q, Yuping H (2008) Screening of bioflocculant-producing strain by ion implantation and flocculating characteristics of bioflocculants. Plasma Sci Technol 10:394. doi:10.1088/1009-0630/10/3/26
Prasertsan P, Wichienchot S, Doelle H, Kennedy JF (2008) Optimization for biopolymer production by Enterobacter cloacae WD7. Carbohyd Polym 71:468–475. doi:10.1016/j.carbpol.2007.06.017
Richard A, Margaritis A (2003) Rheology, oxygen transfer, and molecular weight characteristics of poly(glutamic acid) fermentation by Bacillus subtilis. Biotechnol Bioeng 82:299–305. doi:10.1002/bit.10568
Salehizadeh H, Yan N (2014) Recent advances in extracellular biopolymer flocculants. Biotechnol Adv 32:1506–1522. doi:10.1016/j.biotechadv.2014.10.004
Shih IL, Van YT (2001) The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresource Technol 79:207–225. doi:10.1016/S0960-8524(01)00074-8
Shih L, Wu JY (2009) Biosynthesis and application of poly (γ-glutamic acid). In: Bernd R (ed) Microbial production of biopolymers and polymer precursors: applications and perspectives. Horizon Scientific Press, Norfolk, pp. 101–141
Shih IL, Wu PJ, Shieh CJ (2005) Microbial production of a poly(γ-glutamic acid) derivative by Bacillus subtilis. Process Biochem 40:2827–2832. doi:10.1016/j.procbio.2004.12.009
Su X, Shen X, Ding L, Yokota A (2012) Study on the flocculability of the Arthrobacter sp., an actinomycete resuscitated from the VBNC state. World J Microb Biot 28:91–97. doi:10.1007/s11274-011-0795-2
Subramanian SB, Yan S, Tyagi RD, Surampalli RY (2009) Bioflocculants. In: Tyagi RD, Surampalli RY, Yan S, Zhang TC, Kao CM, Lohani BN (eds) Sustainable sludge management. American Society of Civil Engineers, Reston, pp. 146–167
Wang X, Zhang Y, Zhong W (2008) Poly-γ-glutamic acid production by novel isolated Bacillus subtilis zjutzy and its flocculating character. J Biotechnol 136:S43
Wei W, Fang M, Xiuli Y, Aijie W (2008) Purification and characterization of compound bioflocculant. In: The 2nd international conference on bioinformatics and biomedical engineering. Shanghai, IEEE, pp. 1127–1130. doi:10.1109/ICBBE.2008.275
Wong YS, Ong SA, Teng TT, Aminah LN, Kumaran K (2012) Production of bioflocculant by Staphylococcus cohnii sp. from palm oil mill effluent (POME). Water Air Soil Poll 223:3775–3781. doi:10.1007/s11270-012-1147-z
Wu JY, Ye HF (2007) Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem 42:1114–1123. doi:10.1016/j.procbio.2007.05.006
Xu H, Jiang M, Li H, Lu D, Ouyang P (2005) Efficient production of poly(γ-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem 40:519–523. doi:10.1016/j.procbio.2003.09.025
Yang X (2011) Preparation and characterization of γ-poly (glutamic acid) copolymer with glycol diglycidyl ether. Procedia Environmental Sciences 8:11–15. doi:10.1016/j.proenv.2011.10.004
Yokoi H, Arima T, Hirose J, Hayashi S, Takasaki Y (1996) Flocculation properties of poly([γ]-glutamic acid) produced by Bacillus subtilis. J Ferment Bioeng 82:84–87. doi:10.1016/0922-338X(96)89461-X
Yokoi H, Natsuda O, Hirose J, Hayashi S, Takasaki Y (1995) Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90. J Ferment Bioeng 79:378–380. doi:10.1016/0922-338X(95)94000-H
Yu W, Chen Z, Shen L, Wang Y, Li Q, Yan S, Zhong CJ, He N (2015) Proteomic profiling of Bacillus licheniformis reveals a stress response mechanism in the synthesis of extracellular polymeric flocculants. Biotechnol Bioeng 9999:1–10. doi:10.1002/bit.25838
Zhang H, Zhu J, Zhu X, Cai J, Zhang A, Hong Y, Huang J, Huang L, Xu Z (2012) High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresource Technol 116:241–246. doi:10.1016/j.biortech.2011.11.085
Zulkeflee Z, Aris AZ, Shamsuddin ZH, Yusoff MK (2012) Cation dependence, pH tolerance, and dosage requirement of a bioflocculant produced by Bacillus spp. UPMB13: flocculation performance optimization through kaolin assays. Sci World J. doi:10.1100/2012/495659
Acknowledgments
This research has been funded by the Spanish Ministerio de Economia y Competitividad (project CTM2015-69513-R). Dimitrios Komilis thanks Tecniospring for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulkeflee, Z., Shamsuddin, Z.H., Aris, A.Z. et al. Glutamic Acid Independent Production of Bioflocculants by Bacillus subtilis UPMB13. Environ. Process. 3, 353–367 (2016). https://doi.org/10.1007/s40710-016-0161-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0161-3