Abstract
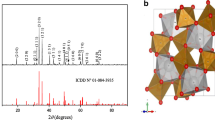
The spinel CuFe2O4, prepared by nitrate route, is successfully tested for Ni2+ reduction under solar light. CuFe2O4 is a narrow band gap semiconductor with a direct optical transition at 1.66 eV. The electro-kinetic parameters indicate a good electrochemical stability with a high O2-over voltage. The energy diagram shows the feasibility of CuFe2O4 for Ni2+ deposition under illumination. At pH ~ 7, the conduction band (−1.22 V SCE ) is located below the Ni2+ level (−0.60 V SCE ), leading to a spontaneous nickel reduction under solar light. The charge transfer is mediated via TiO2. The photocatalytic performance is optimized with respect to the Ni2+ concentration and the catalyst dose. 72 % of Ni2+ (30 ppm) are reduced after ~5 h of exposure to sunlight (115 mW cm−2) on the hetero-system CuFe2O4/TiO2 (mass ratio = 1/1). The photochemical hydrogen evolution, an issue of energy concern, occurs competitively on ultra fine Ni aggregates. The improved performance {0.43 cm3 mn−1 (g catalyst)−1} is due to intimate contact of Ni/TiO2 and low over-voltage on nickel clusters.











Similar content being viewed by others
Notes
Measured with a capacimeter (G w Instek LCR-819) at a fixed frequency 10 kHz.
Reference
Alexéev V, (1980) Analyse Quantitative. Edition Mir Moscou
Boumaza S, Bouguelia A, Bouarab R, Trari M (2009) Physical and photoelectrochemical studies for hydrogen photo-evolution over the spinel ZnCr2O4. Int J Hydrog Energy 34:4963–4967
Brahimi R, Bessekhouad Y, Bouguelia A, Trari M (2008) Improvement of eosin visible light degradation using PbS-sensititized TiO2 light degradation using PbS-sensititized TiO2. J Photochem Photobiol A Chem 194:173–180
Casbeer E, Sharma V-K, Li X-Z (2012) Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep Purif Technol 87:1–14
Chen L-C, Wang C-C, Lu S-W (2013) Annealing effects of sputtered Cu2O nanocolumns on ZnO-coated glass substrate for solar cell applications. J Nanomater 2013:1–6
Dengwei J, Liejin G (2007) WS2 sensitized mesoporous TiO2 for efficient photocatalytic hydrogen production from water under visible light irradiation. Catal Commun 8:795–799
Derbal A, Omeiri S, Bouguelia A, Trari M (2008) Characterization of new heterosystem CuFeO2/SnO2 application to visible-light induced hydrogen evolution. Int J Hydrog Energy 33:4274–4282
Di Paola A, Ikeda S, Marcì G, Ohtani B, Palmisano L (2001) Transition metal doped TiO2: physical properties and photocatalytic behavior. Int J Photoenergy 3:171–176
Kebir M, Chabani M, Nasrallah N, Bensmaili A, Trari M (2011) Coupling adsorption with photocatalysis process for the Cr(VI) removal. Desalination 270:166–173
Kelishadi R (2012) Environmental pollution: health effects and operational implications for pollutants removal. J Environ Public Health 2012:1–2
Kezzim A, Nasrallah N, Abdi A, Trari M (2011) Visible light induced hydrogen on the novel hetero-system CuFe2O4/TiO2. Energy Convers Manag 52:2800–2806
Kiilunen M (1997) Occupational exposure to chromium and nickel in the 1980s in Finland. Sci Total Environ 199:91–101
Kurniawan T-A, Chan G-Y-S, Lo W-H, Babel S (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98
Mishra V, Balomajumder C, Agarwal VK (2013) Adsorption of Cu (II) on the surface of nonconventional biomass: a study on forced convective mass transfer in packed bed column. J Waste Manag 2013:1–8
Omeiri S, Gabès Y, Bouguelia A, Trari M (2008) Photoelectrochemical characterization of the delafossite CuFeO2: application to removal of divalent metals ions. J Electroanal Chem 614:31–40
Preethi V, Kanmani S (2012) Photocatalytic hydrogen production over CuGa2−xFexO4 spinel. Int J Hydrog Energy 37:18740–18746
Protsenko V-S, Kityk A-A, Danilov F-I (2011) Electroreduction of Cr (III) ions in methane sulphonate Solution on Pb Electrode. E-J Chem 8:1714–1719
Rekhila G, Bessekhouad Y, Trari M (2013) Visible light hydrogen production on the novel ferrite NiFe2O4. Int J Hydrog Energy 38:1–14
Rekhila G, Bessekhouad Y, Trari M (2015) Hydrogen evolution under visible light over the solid solution NiFe2−xMnxO4 prepared by sol gel. Int J Hydrog Energy 40:12611–12618
Robbert J, Alzieu J (2005) Batteries. Nickel oxide batteries Ing Tech D3353
Schaumlöffel D (2012) Nickel species: Analysis and toxic effects. J Trace Elem Med Biol 26:1–6
Takimoto Y, Kitta T, Irie H (2012) Visible-light sensitive hydrogen evolution photocatalyst ZnRh2O4. Int J Hydrog Energy 37:134–138
Tanaka Y, Moon S-H, Nikonenko V-V, Xu T (2012) Ion-exchange membranes. Int J Chem Eng 2012:1–3
Weilong W, Xiaobo F (2013) Efficient removal of Cr(VI) with Fe/Mn mixed metal oxide nanocomposites synthesized by a grinding method. J Nanomater 2013:1–8
Acknowledgments
The authors would like to thank Dr. A. Hadibi for technical assistance and Dr. M. Kebir for UV-Visible measurements. They are grateful to the Faculty of Chemistry for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fedailaine, M., Bellal, B., Berkani, S. et al. Photo-electrochemical Characterization of the Spinel CuFe2O4: Application to Ni2+ Removal under Solar Light. Environ. Process. 3, 387–396 (2016). https://doi.org/10.1007/s40710-016-0142-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0142-6