Abstract
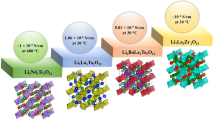
The increasing demand for higher energy density in energy storage systems has instituted the need for electrodes with higher specific capacity and long-term cyclability. However, conventional Li-ion batteries using liquid electrolytes are incapable of reaching the high energy density requirements due to their incompatibility with these high-capacity electrodes. Moreover, these conventional Li-ion batteries are prone to catch fire due to dendrite growth, interfacial instabilities, liquid leakage, and thermal runaway. In contrast, solid-state batteries (SS-LIBs) are a promising technology which can utilize high theoretical specific capacity anodes such as Li metal and Si-based anodes. SS-LIBs experiences internal stresses in between the layers (of electrodes and electrolyte). During lithiation cycling in a SS-LIB, the electrode layers undergo volumetric, phase, and/or lattice dimensional changes, which induces the structural constraints at the interfaces. These constraints raise internal stresses in different stress concentrated regions, leading to poor interfacial contacts, capacity fading, and possible battery failure. In addition, the practicability of SS-LIBs relies on overcoming the scalability challenges, notably, in handling multilayered structures, homogeneity of the interfacial contact, and a suitable stacking process without damaging the multilayered SS-LIB structure. Herein, we evaluate and discuss the cause and impact of these stress management and manufacturability challenges and provide a review of the studies conducted in tackling these challenges. We aim to guide the readers towards tackling these problems and provide possible shortcomings in these solutions.























Similar content being viewed by others
References
Cheng, X., Zhang, R., Zhao, C., & Zhang, Q. (2017). Toward safe lithium metal anode in rechargeable batteries: A review. Chemical Reviews, 117(15), 10403–10473.
Wang, R., Cui, W., Chu, F., & Wu, F. (2020). Lithium metal anodes: Present and future. Journal of Energy Chemistry, 48, 145–159.
Lin, D., Liu, Y., & Cui, Y. (2017). Reviving the lithium metal anode for high-energy batteries. Nature Nanotechnology, 12(3), 194–206.
Zhang, S. (2007). A review on the separators of liquid electrolyte Li-ion batteries. Journal of Power Sources, 164(1), 351–364.
He, P., Chen, Q., Yan, M., Xu, X., Zhou, L., Mai, L., & Nan, C. (2019). Building better zinc-ion batteries: A materials perspective. EnergyChem, 1(3), 100022.
Strauss, F., Teo, J. H., Schiele, A., Bartsch, T., Hatsukade, T., Hartmann, P., Janek, J., & Brezesinski, T. (2020). Gas evolution in lithium-ion batteries: Solid versus liquid electrolyte. ACS Applied Materials & Interfaces, 12(18), 20462–20468.
Yin, Y., Wang, Q., Yang, J., Li, F., Zhang, G., Jiang, C., Mo, H., Yao, J., Wang, K., Zhou, F., Ju, H., & Yao, H. (2020). Metal chloride perovskite thin film based interfacial layer for shielding lithium metal from liquid electrolyte. Nature Communications. https://doi.org/10.1038/s41467-020-15643-9
Yuan, M., & Liu, K. (2020). Rational design on separators and liquid electrolytes for safer lithium-ion batteries. Journal of Energy Chemistry, 43, 58–70.
Pfaffenhuber, C., Göbel, M. C., Popovic, J., & Maier, J. (2013). Soggy-sand electrolytes: Status and perspectives. Physical Chemistry Chemical Physics, 15(42), 18318.
Krauskopf, T., Richter, F., Zeier, W. G., & Janek, J. (2020). Physicochemical concepts of the lithium metal anode in solid-state batteries. Chemical Reviews, 120(15), 7745–7794.
Liu, Y., Liu, Q., Xin, L., Liu, Y., Yang, F., & Stach, E. A. (2017). Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nature Energy, 2(7), 17083.
Aurbach, D., Zinigrad, E., Teller, H., Cohen, Y., Salitra, G., Yamin, H., Dan, P., & Elster, E. A. (2002). Attempts to improve the behavior of Li electrodes in rechargeable lithium batteries. Journal of the Electrochemical Society, 149(10), A1267.
Alexander, G. J., Kamakshy, S. I., & Murugan, R. (2020). Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochimica Acta, 332, 135511.
Xu, W., Wang, J., Ding, F., Chen, X., Nasybulin, E., Zhang, Y., & Zhang, J. (2014). Lithium metal anodes for rechargeable batteries. Energy and Environmental Science, 7(2), 513–537.
Liao, D., Kuang, X., Xiang, J., & Wang, X. (2018). A silicon anode material with layered structure for the lithium-ion battery. Journal of Physics., 986, 012024.
Tarascon, J., & Armand, M. (2001). Issues and challenges facing rechargeable lithium batteries. Nature, 414(6861), 359–367.
Panasonic Intellectual Property Management Co., Ltd. (Osaka)., Sasaki, I., & Honda, K. (2020). Solid electrolyte material including sulfide layer and oxide layer, and battery incorporating the solid electrolyte material. US20190081352.
SDI - Technology | Samsung SDI. Samsungsdi.com. (2020). https://www.samsungsdi.com/column/technology/gallery.html.
Quantumscape Corp., Chen, Z., Donnelly, N., Holme, T., & Singh, D. (2017). Solid electrolyte separator bonding agent. US20170331092A1.
Ionic Materials Inc., & Zimmerman, M. (2020). Solid electrolyte high energy battery. US10741877B1, 11th August 2020. US.
Ionic Materials Inc., Zimmerman, M., & Leising, R. (2020). Lithium metal battery with solid polymer electrolyte. US20200168951.
Sion Power introducing 17 Ah, 810 Wh/L, 400 Wh/kg lithium-metal battery for EVs. Green Car Congress. (2021). https://www.greencarcongress.com/2021/08/20210824-sion.html
Homann, G., Stolz, L., Gerbaldi, C., Laskovic, I. C., Winter, M., & Kasnatscheew, J. (2020). Poly(ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: Evaluating the role of electrolyte oxidation in rapid cell failure. Scientific Reports, 10(1), 4390.
Solid-electrolyte battery developed by Hydro-Québec’s Center of Excellence. Hydroquebec.com. (2021). at https://www.hydroquebec.com/ce-transportation-electrification-energy-storage/solid-electrolyte-batteries.html
How High-Content Silicon Anodes Can Resha–e the EV Landscape - Solid Power. (2021). Solid Power. https://solidpowerbattery.com/high-content-silicon-anode
Core Technologies - ProLogium Technology Co., Ltd. ProLogium Technology Co., Ltd. (2021). https://prologium.com/tech/core-technology/
Frazelle, J. (2021). Battery day. Communications of the ACM, 64(5), 52–59.
Dirican, M., Yan, C., Zhu, P., & Zhang, X. (2019). Composite solid electrolytes for all-solid-state lithium batteries. Materials Science and Engineering R, 136, 27–46.
Schnell, J., Günther, T., Knoche, T., Vieider, C., Köhler, L., Just, A., Keller, M., Khademhosseini, A., & Reinhart, G. (2018). All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. Journal of Power Sources, 382, 160–175.
Jiang, Z., Wang, H., Chen, X., Yang, W., Yao, X., Hu, X., & Han, Q. (2020). Tape‐casting Li0.34La0.56TiO3 ceramic electrolyte films permit high energy density of lithium‐metal batteries. Advanced Materials, 32(6), 1906221.
Hoey, J., Lutfurakhmanov, A., Schulz, D. L., & Akhatov, I. (2012). A review on aerosol-based direct-write and its applications for microelectronics. Journal of Nanotechnology, 2012, 324380.
Chen, C., Yu, T., Yang, M., Huang, X., & Shen, X. (2019). An all-solid-state rechargeable chloride ion battery. Advanced Science, 6(6), 1802130.
Zhang, X., Liu, T., Zhang, S., Huang, X., Xu, B., Lin, Y., Xu, B., Li, L., Nan, C., & Shen, Y. (2017). Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. Journal of the American Chemical Society, 139(39), 13779–13785.
Yoon, K., Lee, S., Oh, K., & Kang, K. (2021). Challenges and strategies towards practically feasible solid-state lithium metal batteries. Advanced Materials, 34(4), 2104666.
He, Y., Lu, C., Liu, S., Zheng, W., & Luo, J. (2019). Interfacial incompatibility and internal stresses in all-solid-state lithium ion batteries. Advanced Energy Materials, 9(36), 1901810.
Zhu, Y., He, X., & Mo, Y. (2015). Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations. ACS Applied Materials & Interfaces, 7(42), 23685–23693.
Bower, A. F., Guduru, P. R., & Sethuraman, V. A. (2011). A finite strain model of stress, diffusion, plastic flow, and electrochemical reactions in a lithium-ion half-cell. Journal of the Mechanics and Physics of Solids, 59(4), 804–828.
McDowell, M. T., Xia, S., & Zhu, T. (2016). The mechanics of large-volume-change transformations in high-capacity battery materials. Extreme Mechanics Letters, 9, 480–494.
Mukhopadhyay, A., & Sheldon, B. W. (2014). Deformation and stress in electrode materials for Li-ion batteries. Progress in Materials Science, 63, 58–116.
Zeng, Z., Liu, N., Zeng, Q., Lee, S., Mao, W. L., & Cui, Y. (2016). In situ measurement of lithiation-induced stress in silicon nanoparticles using micro-Raman spectroscopy. Nano Energy, 22, 105–110.
Chen, A., Qu, C., Shi, Y., & Shi, F. (2020). Manufacturing strategies for solid electrolyte in batteries. Frontiers in Energy Research. https://doi.org/10.3389/fenrg.2020.571440
Hatzell, K. B., & Zheng, Y. (2021). Prospects on large-scale manufacturing of solid state batteries. MRS Energy & Sustainability, 8(1), 33–39.
Liu, Y., Zhang, R., Wang, J., & Wang, Y. (2021). Current and future lithium-ion battery manufacturing. IScience, 24(4), 102332.
Doux, J., Nguyen, H., Tan, D. J. H., Banerjee, A., Wang, X., Wu, E. A., Jo, C., Yang, H., & Meng, Y. S. (2019). Stack pressure considerations for room-temperature all-solid-state lithium metal batteries. Advanced Energy Materials, 10(1), 1903253.
Yu, H., Taha, D. M., Thompson, T., Taylor, N. G., Drews, A. R., Sakamoto, J., & Thornton, K. (2019). Deformation and stresses in solid-state composite battery cathodes. Journal of Power Sources, 440, 227116.
Tian, H., Chakraborty, A., Talin, A. A., Eisenlohr, P., & Qi, Y. (2020). Evaluation of the electrochemo-mechanically induced stress in all-solid-state li-ion batteries. Journal of the Electrochemical Society, 167(9), 090541.
Li, W., Xia, Y., Sahraei, E., & Luo, H. (2018). State-of-charge dependence of mechanical response of lithium-ion batteries: A result of internal stress. Journal of the Electrochemical Society, 165(7), A1537–A1546.
Chen, J. M., Wu, J., Wang, X., Zhou, A., & Yang, Z. (2021). Research progress and application prospect of solid-state electrolytes in commercial lithium-ion power batteries. Energy Storage Materials, 35, 70–87.
Abakumov, A. M., Fedotov, S. S., Antipov, E. V., & Tarascon, J. (2020). Solid state chemistry for developing better metal-ion batteries. Nature Communications, 11(1), 4976.
Zheng, Y., Yao, Y., Ou, J., Li, M., Luo, D., Dou, H., Li, Z., Amine, K., Yu, A., & Chen, Z. (2020). A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chemical Society Reviews, 49(23), 8790–8839.
Kerman, K., Luntz, A. C., Viswanathan, V., Chiang, Y., & Chen, Z. (2017). Review—Practical challenges hindering the development of solid state Li ion batteries. Journal of the Electrochemical Society, 164(7), A1731–A1744.
Zhang, S. (2017). Chemomechanical modeling of lithiation-induced failure in high-volume-change electrode materials for lithium ion batteries. Npj Computational Materials. https://doi.org/10.1038/s41524-017-0009-z
Zhang, F., Huang, Q., Tang, Z., Li, A., Shao, Q., Zhang, L., Li, X., & Zhang, J. (2020). A review of mechanics-related material damages in all-solid-state batteries: Mechanisms, performance impacts and mitigation strategies. Nano Energy, 70, 104545.
Ren, Y., & Hatzell, K. B. (2021). Elasticity-oriented design of solid-state batteries: challenges and perspectives. Journal of Materials Chemistry A Materials for Energy and Sustainability, 9(24), 13804–13821.
Kato, A., Yamamoto, M., Sakuda, A., Hayashi, A., & Tatsumisago, M. (2018). Mechanical properties of Li2S–P2S5 glasses with lithium halides and application in all-solid-state batteries. ACS Applied Energy Materials, 1(3), 1002–1007.
Kim, D., Lee, H. C., Song, Y. S., Park, J. Y., Lee, S. Y., & Jung, Y. S. (2019). Sheet-type Li6PS5Cl-infiltrated Si anodes fabricated by solution process for all-solid-state lithium-ion batteries. Journal of Power Sources, 426, 143–150.
Hu, J., Sun, Z., Gao, Y., Li, P., Wu, Y., Chen, S., Wang, R., Li, N., Yang, W., Shen, Y., & Bo, S. (2022). 3D stress mapping reveals the origin of lithium-deposition heterogeneity in solid-state lithium-metal batteries. Cell Reports Physical Science, 3(7), 100938.
Han, S. W., Lee, C. H., Lewis, J. S., Yeh, D., Liu, Y., Lee, H., & McDowell, M. T. (2021). Stress evolution during cycling of alloy-anode solid-state batteries. Joule, 5(9), 2450–2465.
McConohy, G., Xu, X., Cui, T., Barks, E., Wang, S., Kaeli, E., Melamed, C., Gu, X., & Chueh, W. C. (2023). Mechanical regulation of lithium intrusion probability in garnet solid electrolytes. Nature Energy, 8(3), 241–250.
Ramesh, K. P., Winie, T., & Arof, A. K. (2010). Mechanical studies on poly(vinyl chloride)–poly(methyl methacrylate)-based polymer electrolytes. Journal of Materials Science, 45(5), 1280–1283.
Hatzell, K. B. (2020). Not all lithium filaments are the same in solid-state batteries. Joule, 4(4), 719–721.
Deng, Z., Wang, Z., Chu, I.-H., Luo, J., & Ong, S. P. (2015). Elastic properties of alkali superionic conductor electrolytes from first principles calculations. Journal of the Electrochemical Society, 163(2), A67–A74.
Chen-Yang, Y., Chen, H., Lin, F., & Chen, C. (2002). Polyacrylonitrile electrolytes: 1. A novel high-conductivity composite polymer electrolyte based on PAN, LiClO4 and α-Al2O3. Solid State Ionics, 150, 327–335.
Ramesh, S., Winie, T., & Arof, A. K. (2010). Mechanical studies on poly(vinyl chloride)–poly(methyl methacrylate)-based polymer electrolytes. Journal of Materials Science, 45, 1280–1283.
Chung, N. K., Kwon, Y. D., & Kim, D. (2003). Thermal, mechanical, swelling, and electrochemical properties of poly(vinylidene fluoride)-co-hexafluoropropylene/poly(ethylene glycol) hybrid-type polymer electrolytes. Journal of Power Sources, 124, 148–154.
Ramesh, S., Winie, T., & Arof, A. (2007). Investigation of mechanical properties of polyvinyl chloride–polyethylene oxide (PVC–PEO) based polymer electrolytes for lithium polymer cells. European Polymer Journal, 43, 1963–1968.
Sakuda, A., Hayashi, A., & Tatsumisago, M. (2013). Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Scientific Reports, 3, 2261.
Kim, Y., Jo, H., Allen, J. L., Choe, H., Wolfenstine, J., Sakamoto, J., & Pharr, G. (2016). The effect of relative density on the mechanical properties of hot-pressed cubic Li7La3Zr2O12. J. Am. Ceram., 99, 1367–1374.
Jackman, S. D., & Cutler, R. A. (2012). Effect of microcracking on ionic conductivity in LATP. Journal of Power Sources, 218, 65–72.
Cho, Y.-H., Wolfenstine, J., Rangasamy, E., Kim, H., Choe, H., & Sakamoto, J. (2012). Mechanical properties of the solid Li-ion conducting electrolyte: Li0.33La0.57TiO3. Journal of Materials Science, 47, 5970–5977.
Herbert, E. G., Tenhaeff, W. E., Dudney, N. J., & Pharr, G. M. (2011). Mechanical characterization of LiPON films using nanoindentation. Thin Solid Films, 520(1), 413–418.
Wang, Z., Wu, M., Liu, G., Lei, X., Xu, B., & Ouyang, C. (2014). Elastic properties of new solid state electrolyte material Li10GeP2S12: A study from first-principles calculations. International Journal of Electrochemical Science, 9, 562–568.
Manthiram, A., Yu, X., & Wang, S. (2017). Lithium battery chemistries enabled by solid-state electrolytes. Nature Reviews Materials, 2(4), 16103.
Xia, S., Wu, X., Zhang, Z., Cui, Y., & Liu, W. (2019). Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem, 5(4), 753–785.
Dixit, M. B., Zaman, W., Hortance, N., Vujic, S., Harkey, B. A., Shen, F., Tsai, W. C., De Andrade, V., Chen, X. R., Balke, N., & Hatzell, K. B. (2020). Nanoscale mapping of extrinsic interfaces in hybrid solid electrolytes. Joule, 4(1), 207–221.
Wang, M. J., Choudhury, R., & Sakamoto, J. (2019). Characterizing the Li–solid–electrolyte interface dynamics as a function of stack pressure and current density. Joule, 3(9), 2165–2178.
Athanasiou, C. E., Jin, M. Y., Ramirez, C. M., Padture, N. P., & Sheldon, B. W. (2020). High-toughness inorganic solid electrolytes via the use of reduced graphene oxide. Matter, 3(1), 212–229.
Porz, L., Swamy, T., Sheldon, B. W., Rettenwander, D., Frömling, T., Thaman, H. L., Berendts, S., Uecker, R., Carter, W. C., & Chiang, Y. (2017). Mechanism of lithium metal penetration through inorganic solid electrolytes. Advanced Energy Materials, 7(20), 1701003.
Shen, F., Dixit, M. B., Xiao, X., & Hatzell, K. B. (2018). Effect of pore connectivity on Li dendrite propagation within LLZO electrolytes observed with synchrotron X-ray tomography. ACS Energy Letters, 3(4), 1056–1061.
Kazyak, E., Garcia-Mendez, R., LePage, W. S., Sharafi, A., Davis, A. M., Sanchez, A. A., Chen, K., Haslam, C. G., Sakamoto, J., & Dasgupta, N. P. (2020). Li penetration in ceramic solid electrolytes: Operando microscopy analysis of morphology, propagation, and reversibility. Matter, 2(4), 1025–1048.
Doux, J., Yang, Y., Tan, D. J. H., Nguyen, H., Wu, E. A., Wang, X., Banerjee, A., & Meng, Y. S. (2020). Pressure effects on sulfide electrolytes for all solid-state batteries. Journal of Materials Chemistry A Materials for Energy and Sustainability, 8(10), 5049–5055.
Dixit, M. B., Zaman, W., Bootwala, Y., Zheng, Y., Hatzell, M. C., & Hatzell, K. B. (2019). Scalable Manufacturing of Hybrid Solid Electrolytes with Interface Control. ACS Applied Materials & Interfaces, 11(48), 45087–45097.
He, Y., Chen, S., Nie, L., Sun, Z., Wu, X., & Liu, W. (2020). Stereolithography three-dimensional printing solid polymer electrolytes for all-solid-state lithium metal batteries. Nano Letters, 20(10), 7136–7143.
Baranowski, L. L., Heveran, C. M., Ferguson, V. L., & Stoldt, C. R. (2016). Multi-scale mechanical behavior of the Li3PS4 solid-phase electrolyte. ACS Applied Materials & Interfaces, 8(43), 29573–29579.
McGrogan, F. P., Swamy, T., Bishop, S. R., Eggleton, E., Porz, L., Chen, X., Chiang, Y., & Van Vliet, K. J. (2017). Compliant yet brittle mechanical behavior of Li 2 S-P 2 S 5 lithium-ion-conducting solid electrolyte. Advanced Energy Materials, 7(12), 1602011.
Shi, T., Tu, Q., Tian, Y., Xiao, Y., Miara, L. J., Kononova, O., & Ceder, G. (2020). High active material loading in all-solid-state battery electrode via particle size optimization. Advanced Energy Materials, 10(1), 1902881.
Chi, M., Wang, H., Pandian, A. S., Peng, R., Gilroy, K. D., Liang, C., & Xia, Y. (2018). Fabrication of sub-micrometer-thick solid electrolyte membranes of β-Li 3 PS 4 via tiled assembly of nanoscale, plate-like building blocks. Advanced Energy Materials, 8(21), 1800014.
Kanno, R., & Murayama, M. (2001). Lithium ionic conductor Thio-LISICON: the Li[sub 2]S-GeS[sub 2]-P[sub 2]S[sub 5] system. Journal of the Electrochemical Society, 148(7), A742.
Lee, Y., Fujiki, S., Jung, C., Suzuki, N., Yashiro, N., Omoda, R., Ko, D., Shiratsuchi, T., Sugimoto, T., Ryu, S., Ku, J. H., Watanabe, T., Park, Y., Aihara, Y., Im, D., & Han, I. W. (2020). High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nature Energy, 5(4), 299–308.
Wang, M. J., Kazyak, E., Dasgupta, N. P., & Sakamoto, J. (2021). Transitioning solid-state batteries from lab to market: Linking electro-chemo-mechanics with practical considerations. Joule, 5(6), 1371–1390.
Tikekar, M. D., Choudhury, S., Tu, Z., & Archer, L. A. (2016). Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nature Energy, 1(9), 16114.
Kasemchainan, J., Zekoll, S., Jolly, D. S., Ning, Z., Hartley, G. O., Marrow, T. J., & Bruce, P. G. (2019). Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nature Materials, 18(10), 1105–1111.
Han, F., Yue, J., Zhu, X., & Wang, C. (2018). Suppressing Li dendrite formation in Li 2 S-P 2 S 5 solid electrolyte by LiI Incorporation. Advanced Energy Materials, 8(18), 1703644.
Liu, Y., Guo, K., Wang, C., & Gao, H. (2019). Wrinkling and ratcheting of a thin film on cyclically deforming plastic substrate: Mechanical instability of the solid-electrolyte interphase in Li–ion batteries. Journal of the Mechanics and Physics of Solids, 123, 103–118.
Lau, J. C., DeBlock, R. H., Butts, D. M., Ashby, D. S., Choi, C. Y., & Dunn, B. (2018). Sulfide solid electrolytes for lithium battery applications. Advanced Energy Materials, 8(27), 1800933.
Whiteley, J. M., Taynton, P., Zhang, W., & Lee, S. (2015). Ultra-thin solid-state li-ion electrolyte membrane facilitated by a self-healing polymer matrix. Advanced Materials, 27(43), 6922–6927.
Tan, D. J. H., Banerjee, A., Deng, Z., Wu, E. A., Nguyen, H., Doux, J., Wang, X., Cheng, J., Ong, S. P., Meng, Y. S., & Chen, Z. (2019). Enabling thin and flexible solid-state composite electrolytes by the scalable solution process. ACS Applied Energy Materials, 2(9), 6542–6550.
Li, Y., Wang, X., Zhou, H., Xing, X., Banerjee, A., Holoubek, J., Liu, P., & Meng, Y. S. (2020). Thin solid electrolyte layers enabled by nanoscopic polymer binding. ACS Energy Letters, 5(3), 955–961.
Lei, W., Jiao, X., Yang, S., Ajdari, F. B., Salavati-Niasari, M., Feng, Y., Yin, J., Ungar, G., & Song, J. (2022). Temperature and stress-resistant solid state electrolyte for stable lithium-metal batteries. Energy Storage Materials, 49, 502–508.
Zhao, Q., Liu, X., Stalin, S., Khan, K., & Archer, L. A. (2019). Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nature Energy, 4(5), 365–373.
Lee, M. D., Han, J., Lee, K., Lee, Y. H., Kim, B. Y., Jung, K., Kim, B. J., & Lee, S. H. (2022). Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature, 601(7892), 217–222.
Deng, C., Chen, N., Hou, C., Liu, H., Zhou, Z., & Chen, R. (2021). Enhancing interfacial contact in solid-state batteries with a gradient composite solid electrolyte. Small (Weinheim an der Bergstrasse, Germany), 17(18), 2006578.
Feng, X., Ouyang, M., Liu, X., Lu, L., Xia, Y., & He, X. (2018). Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Materials, 10, 246–267.
Gabrisch, H., Wilcox, J. D., & Doeff, M. M. (2008). TEM study of fracturing in spherical and plate-like LiFePO[sub 4] particles. Electrochemical and Solid State Letters, 11(3), A25.
Zaman, W., Hortance, N., Dixit, M. B., De Andrade, V., & Hatzell, K. B. (2019). Visualizing percolation and ion transport in hybrid solid electrolytes for Li–metal batteries. Journal of Materials Chemistry, Materials for Energy and Sustainability, 7(41), 23914–23921.
Zhang, X., Xiang, Q., Tang, S., Wang, A., Liu, X., & Luo, J. (2020). Long cycling life solid-state Li metal batteries with stress self-adapted Li/garnet interface. Nano Letters, 20(4), 2871–2878.
Wang, X., Zeng, W., Hong, L., Xu, W., Yang, H., Wang, F., Duan, H., Tang, M., & Jiang, H. (2018). Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nature Energy, 3(3), 227–235.
Baggetto, L., Niessen, R. R., Roozeboom, F., & Notten, P. P. (2008). High energy density all-solid-state batteries: A challenging concept towards 3D integration. Advanced Functional Materials, 18(7), 1057–1066.
Liu, X., Zhang, L., Zhong, L., Liu, Y., Zheng, H., Wang, J., Cho, J., Dayeh, S. A., Picraux, S. T., Sullivan, J. L., Mao, S. X., Ye, Z., & Huang, J. (2011). Ultrafast electrochemical lithiation of individual Si nanowire anodes. Nano Letters, 11(6), 2251–2258.
Wang, C. M., Li, X., Wang, Z., Xu, W., Liu, J., Gao, F., Kovarik, L., Zhang, J. G., Howe, J. Y., Burton, D. R., Liu, Z., Xiao, X., Thevuthasan, S., & Baer, D. R. (2012). In situ TEM investigation of congruent phase transition and structural evolution of nanostructured silicon/carbon anode for lithium ion batteries. Nano Letters, 12(3), 1624–1632.
Yoon, T., Nguyen, C. C., Seo, D. M., & Lucht, B. L. (2015). Capacity fading mechanisms of silicon nanoparticle negative electrodes for lithium ion batteries. Journal of the Electrochemical Society, 162(12), A2325–A2330.
McDowell, M. T., Lee, S., Harris, J. A., Korgel, B. A., Wang, C., Nix, W. D., & Cui, Y. (2013). In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Letters, 13(2), 758–764.
Gu, M., Li, Y., Li, X., Hu, S. Y., Zhang, X., Xu, W., Thevuthasan, S., Baer, D. R., Zhang, J., Liu, J., & Wang, C. (2012). In situ TEM study of lithiation behavior of silicon nanoparticles attached to and embedded in a carbon matrix. ACS Nano, 6(9), 8439–8447.
Lee, S., McDowell, M. T., Berla, L. A., Nix, W. D., & Cui, Y. (2012). Fracture of crystalline silicon nanopillars during electrochemical lithium insertion. Proceedings of the National Academy of Sciences of the United States of America, 109(11), 4080–4085.
Liu, X., & Huang, J. (2011). In situ TEM electrochemistry of anode materials in lithium ion batteries. Energy and Environmental Science, 4(10), 3844.
Aravindan, V., Jinesh, K. B., Prabhakar, R., Kale, V. S., & Madhavi, S. (2013). Atomic layer deposited (ALD) SnO2 anodes with exceptional cycleability for Li-ion batteries. Nano Energy, 2(5), 720–725.
Misra, S., Liu, N., Nelson, J., Hong, S. B., Cui, Y., & Toney, M. F. (2012). In situ X-ray diffraction studies of (de)lithiation mechanism in silicon nanowire anodes. ACS Nano, 6(6), 5465–5473.
Tan, D. J. H., Chen, Y., Yang, H., Bao, W., Sreenarayanan, B., Doux, J., Li, W., Lu, B., Ham, S., Sayahpour, B., Scharf, J., Wu, E. A., Deysher, G., Han, H. E., Hah, H. J., Jeong, H., Lee, J. M., Chen, Z., & Meng, Y. S. (2021). Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science, 373(6562), 1494–1499.
Yu, S. H., Feng, X., Zhang, N., Seok, J., & Abruña, H. D. (2018). Understanding conversion-type electrodes for lithium rechargeable batteries. Accounts of Chemical Research, 51(2), 273–281.
Bhatt, M. D., & Lee, J. Y. (2019). High capacity conversion anodes in Li-ion batteries: A review. International Journal of Hydrogen Energy, 44(21), 10852–10905.
Oumellal, Y., Rougier, A., Nazri, G., Tarascon, J., & Aymard, L. (2008). Metal hydrides for lithium-ion batteries. Nature Materials, 7(11), 916–921.
Tong, Y., Yang, H., Xiong, T., Adekoya, D., Qiu, W., Wang, Z., Zhang, Q., & Balogun, M. (2020). Adsorption energy engineering of nickel oxide hybrid nanosheets for high areal capacity flexible lithium-ion batteries. Energy Storage Materials, 25, 41–51.
Liu, Y., Wan, H., Jiang, N., Zhang, W., Zhang, H., Chang, B., Wang, Q., Zhang, Y., Wang, Z., Luo, S., & Sun, H. (2019). Chemical reduction-induced oxygen deficiency in Co3O4 nanocubes as advanced anodes for lithium ion batteries. Solid State Ionics, 334, 117–124.
Lee, J. E., Choi, S. H., Im, G., Lee, K. S., Lee, T., Oh, J., Lee, N., Kim, H., Kim, Y., Lee, S., & Choi, J. W. (2022). Room-temperature anode-less all-solid-state batteries via the conversion reaction of metal fluorides. Advanced Materials, 34(40), 2203580.
Lin, F., Nordlund, D., Weng, T., Zhu, Y., Ban, C., Richards, R. M., & Xin, H. L. (2014). Phase evolution for conversion reaction electrodes in lithium-ion batteries. Nature Communications, 5(1), 3358.
Luo, L., Wu, J., Xu, J., & Dravid, V. P. (2014). Atomic resolution study of reversible conversion reaction in metal oxide electrodes for lithium-ion battery. ACS Nano, 8(11), 11560–11566.
Oudenhoven, J. J., Baggetto, L., & Notten, P. P. (2011). All-solid-state lithium-ion microbatteries: A review of various three-dimensional concepts. Advanced Energy Materials, 1(1), 10–33.
Wang, H., Jang, Y., Huang, B., Sadoway, D. R., & Chiang, Y. (1999). Electron microscopic characterization of electrochemically cycled LiCoO2 and Li(Al, Co)O2 battery cathodes. Journal of Power Sources, 81–82, 594–598.
Mu, L., Lin, R., Xu, R., Han, L., Xia, S., Sokaras, D., Steiner, J. F., Weng, T., Nordlund, D., Doeff, M. M., Liu, Y., Zhao, K., Xin, H. L., & Lin, F. (2018). Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Letters, 18(5), 3241–3249.
Inaba, M., Iriyama, Y., Ogumi, Z., Todzuka, Y., & Tasaka, A. (1997). Raman study of layered rock-salt LiCoO2 and its electrochemical lithium deintercalation. Journal of Raman Spectroscopy, 28(8), 613–617.
Reimers, J. N., & Dahn, J. R. (1992). Electrochemical and in situ X-ray diffraction studies of lithium intercalation in Li x CoO2. Journal of the Electrochemical Society, 139(8), 2091–2097.
Tsai, Y. T., Hwang, B., Ceder, G., Sheu, H., Liu, D., & Lee, J. S. H. (2005). In-situ X-ray absorption spectroscopic study on variation of electronic transitions and local structure of LiNi1/3Co1/3Mn1/3O2 cathode material during electrochemical cycling. Chemistry of Materials, 17(12), 3191–3199.
Yoon, W., Chung, K. Y., McBreen, J., & Yang, X. (2006). A comparative study on structural changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during first charge using in situ XRD. Electrochemistry Communications, 8(8), 1257–1262.
Ohzuku, T., Kitagawa, M., & Hirai, T. (1990). Electrochemistry of manganese dioxide in lithium nonaqueous cell: III. X-ray diffractional study on the reduction of spinel-related manganese dioxide. Journal of the Electrochemical Society, 137(3), 769.
Çapraz, Ö. Ö., Bassett, K. L., Gewirth, A. A., & Sottos, N. R. (2017). Electrochemical stiffness changes in lithium manganese oxide electrodes. Advanced Energy Materials, 7(7), 1601778.
Qi, Y., Hector, L. G., James, C., & Kim, K. S. (2014). Lithium concentration dependent elastic properties of battery electrode materials from first principles calculations. Journal of the Electrochemical Society, 161(11), F3010–F3018.
Wang, H., Yang, Y., Liang, Y., Robinson, J. A., Li, Y., Jackson, A., Cui, Y., & Dai, H. (2011). Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Letters, 11(7), 2644–2647.
Gu, W., Magasinski, A., Zdyrko, B., & Yushin, G. (2015). Metal fluorides nanoconfined in carbon nanopores as reversible high capacity cathodes for Li and Li-ion rechargeable batteries: FeF2as an example. Advanced Energy Materials, 5(4), 1401148.
Cabana, J., Monconduit, L., Larcher, D., & Palacín, M. R. (2010). Beyond intercalation-based Li-Ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions (Adv. Mater. 35/2010). Advanced Materials, 22(35), 170–192.
Li, L., Jacobs, R., Feng, Y., Gan, L., Wang, F., Morgan, D., & Jin, S. (2016). Origins of large voltage hysteresis in high-energy-density metal fluoride lithium-ion battery conversion electrodes. Journal of the American Chemical Society, 138(8), 2838–2848.
Fan, X., Zhu, Y., Luo, C. B., Gao, T., Suo, L., Liou, S., Xu, K., & Wang, C. (2016). In situ lithiated FeF3/C nanocomposite as high energy conversion-reaction cathode for lithium-ion batteries. Journal of Power Sources, 307, 435–442.
Pang, Q., & Nazar, L. F. (2016). Long-life and high-areal-capacity Li–S batteries enabled by a light-weight polar host with intrinsic polysulfide adsorption. ACS Nano, 10(4), 4111–4118.
Li, G., Sun, J., Hou, W., Jiang, S., Huang, Y., & Geng, J. (2016). Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium–sulfur batteries. Nature Communications, 7(1), 10601.
Han, K., Liu, Z., Shen, J., Lin, Y., Dai, F., & Ye, H. (2015). A free-standing and ultralong-life lithium-selenium battery cathode enabled by 3D mesoporous carbon/graphene hierarchical architecture. Advanced Functional Materials, 25(3), 455–463.
Zhang, J., Li, Z., & Lou, X. W. D. (2017). A freestanding selenium disulfide cathode based on cobalt disulfide-decorated multichannel carbon fibers with enhanced lithium storage performance. Angewandte Chemie, 56(45), 14107–14112.
Xu, J., Xin, S., Liu, J., Wang, J., Lei, Y., & Yu, S. (2016). Elastic carbon nanotube aerogel meets tellurium nanowires: A binder- and collector-free electrode for Li–Te batteries. Advanced Functional Materials, 26(21), 3580–3588.
He, J., Chen, Y., Lv, W., Wen, K., Wang, Z., Zhang, W., Li, Y., Qin, W., & He, W. (2016). Three-dimensional hierarchical reduced graphene oxide/tellurium nanowires: A high-performance freestanding cathode for Li–Te batteries. ACS Nano, 10(9), 8837–8842.
Zhao, Q., Lu, Y., Zhu, Z., Tao, Z., & Chen, J. (2015). Rechargeable lithium-iodine batteries with iodine/nanoporous carbon cathode. Nano Letters, 15(9), 5982–5987.
Lin, C., Fan, X., Pearse, A. J., Liou, S., Gregorczyk, K., Leskes, M., Wang, C., Lee, S. Y., Rubloff, G. W., & Noked, M. (2017). Highly reversible conversion-type FeOF composite electrode with extended lithium insertion by atomic layer deposition LiPON protection. Chemistry of Materials, 29(20), 8780–8791.
Chevrier, V., Hautier, G., Ong, S. P., Doe, R. E., & Ceder, G. (2013). First-principles study of iron oxyfluorides and lithiation of FeOF. Physical Review B, 87(9), 094118.
Huang, Q., Pollard, T. P., Ren, X., Kim, D., Magasinski, A., Borodin, O., & Yushin, G. (2019). Fading mechanisms and voltage hysteresis in FeF 2 –NiF 2 solid solution cathodes for lithium and lithium-ion batteries. Small (Weinheim an der Bergstrasse, Germany), 15(6), 1804670.
Ohno, S., Koerver, R., Dewald, G., Rosenbach, C., Titscher, P., Steckermeier, D., Kwade, A., Janek, J., & Zeier, W. G. (2019). Observation of chemomechanical failure and the influence of cutoff potentials in all-solid-state Li–S batteries. Chemistry of Materials, 31(8), 2930–2940.
Xu, R., Yue, J., Liu, S., Tu, J., Han, F., Liu, P., & Wang, C. (2019). Cathode-supported all-solid-state lithium-sulfur batteries with high cell-level energy density. ACS Energy Letters, 4(5), 1073–1079.
Xu, Z., Huang, J., Chong, W. H., Qin, X., Wang, X., Zhou, L. M., & Kim, J. K. (2017). In situ TEM study of volume expansion in porous carbon nanofiber/sulfur cathodes with exceptional high-rate performance. Advanced Energy Materials, 7(9), 1602078.
Xu, L., Hu, Y., Zhang, H., Jiang, H., & Li, C. (2016). Confined synthesis of FeS2 nanoparticles encapsulated in carbon nanotube hybrids for ultrastable lithium-ion batteries. ACS Sustainable Chemistry & Engineering, 4(8), 4251–4255.
Ji, L., Rao, M., Zheng, H., Zhang, L., Li, Y., Duan, W., Guo, J., Cairns, E. J., & Zhang, Y. (2011). Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. Journal of the American Chemical Society, 133(46), 18522–18525.
Song, J., Gordin, M. L., Xu, T., Chen, S., Yu, Z., Sohn, H., Lu, J., Ren, Y., Duan, Y., & Wang, D. (2015). Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angewandte Chemie, 54(14), 4325–4329.
Qiu, Y., Li, W., Zhao, W., Li, G., Hou, Y., Liu, M., Zhou, L., Ye, F., Zhi, C., Wei, Z., Yang, S., Duan, W., Ye, Y., Guo, J., & Zhang, Y. (2014). High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Letters, 14(8), 4821–4827.
Scholz, J., Kayaalp, B., Juhl, A. C., Clemens, D. L., Fröba, M., & Mascotto, S. (2018). Severe loss of confined sulfur in nanoporous carbon for Li–S batteries under wetting conditions. ACS Energy Letters, 3(2), 387–392.
Gueon, D., Ju, M., & Moon, J. H. (2020). Complete encapsulation of sulfur through interfacial energy control of sulfur solutions for high-performance Li−S batteries. Proceedings of the National Academy of Sciences of the United States of America, 117(23), 12686–12692.
Han, F., Yue, J., Fan, X., Gao, T., Luo, C. B., Ma, Z., Suo, L., & Wang, C. (2016). High-performance all-solid-state lithium-sulfur battery enabled by a mixed-conductive Li2S nanocomposite. Nano Letters, 16(7), 4521–4527.
Huang, Q., Turcheniuk, K., Ren, X., Magasinski, A., Song, A., Xiao, Y., Kim, D., & Yushin, G. (2019). Cycle stability of conversion-type iron fluoride lithium battery cathode at elevated temperatures in polymer electrolyte composites. Nature Materials, 18(12), 1343–1349.
Lee, K., Lee, J. E., Choi, S., Char, K., & Choi, J. W. (2019). Thiol-ene click reaction for fine polarity tuning of polymeric binders in solution-processed all-solid-state batteries. ACS Energy Letters, 4(1), 94–101.
Oh, J., Choi, S. H., Chang, B., Lee, J. E., Lee, T., Lee, N., Kim, H., Kim, Y., Im, G., Lee, S., & Choi, J. W. (2022). Elastic binder for high-performance sulfide-based all-solid-state batteries. ACS Energy Letters, 7(4), 1374–1382.
Chang, B., Kim, J., Cho, Y., Hwang, I., Jung, M. W., Char, K., Lee, K. E., Kim, K., & Choi, J. W. (2020). Highly elastic binder for improved cyclability of nickel-rich layered cathode materials in lithium-ion batteries. Advanced Energy Materials, 10(29), 2001069.
Choi, S., Kwon, T. G., Coskun, A., & Choi, J. W. (2017). Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science, 357(6348), 279–283.
Chiang, S., Marshall, D. J., & Evans, A. (1982). The response of solids to elastic/plastic indentation. I. Stresses and residual stresses. Journal of Applied Physics, 53(1), 298–311.
Dixit, M. B., Moreno, D. A., Xiao, X., Hatzell, M. C., & Hatzell, K. B. (2019). Mapping charge percolation in flowable electrodes used in capacitive deionization. ACS Materials Letters, 1(1), 71–76.
Robinson, I. K., & Harder, R. (2009). Coherent X-ray diffraction imaging of strain at the nanoscale. Nature Materials, 8(4), 291–298.
Ebner, M., Marone, F., Stampanoni, M., & Wood, V. (2013). Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science, 342(6159), 716–720.
Sottmann, J., Di Michiel, M., Fjellvåg, H., Malavasi, L., Margadonna, S., Vajeeston, P., Vaughan, G., & Wragg, D. S. (2017). Chemical structures of specific sodium ion battery components determined by operando pair distribution function and X-ray diffraction computed tomography. Angewandte Chemie, 56(38), 11385–11389.
LePage, W. S., Chen, Y., Kazyak, E., Chen, K., Sanchez, A. J., Poli, A. A., Arruda, E. M., Thouless, M. D., & Dasgupta, N. P. (2019). Lithium mechanics: Roles of strain rate and temperature and implications for lithium metal batteries. Journal of the Electrochemical Society, 166(2), A89–A97.
Dai, C., Li, C., Huang, H., Wang, Z., Zhu, X., Liao, X., Chen, X., Pan, Y., & Fang, D. (2019). In situ strain measurements and stress analysis of SiO@C composite electrodes during electrochemical cycling by using digital image correlation. Solid State Ionics, 331, 56–65.
Sethuraman, V. A., Chon, M. J., Shimshak, M., Van Winkle, N., & Guduru, P. R. (2010). In situ measurement of biaxial modulus of Si anode for Li-ion batteries. Electrochemistry Communications, 12(11), 1614–1617.
Soni, S. K., Sheldon, B. W., Xiao, X., & Tokranov, A. (2011). Thickness effects on the lithiation of amorphous silicon thin films. Scripta Materialia, 64(4), 307–310.
Soni, S. K., Sheldon, B. W., Xiao, X., Bower, A. F., & Verbrugge, M. W. (2012). Diffusion mediated lithiation stresses in Si thin film electrodes. Journal of the Electrochemical Society, 159(9), A1520–A1527.
Nation, L., Li, J., James, C., Qi, Y., Dudney, N. J., & Sheldon, B. W. (2017). In situ stress measurements during electrochemical cycling of lithium-rich cathodes. Journal of Power Sources, 364, 383–391.
Xie, H., Song, H., Guo, J., Kang, Y., Yang, W., & Zhang, Q. (2019). In situ measurement of rate-dependent strain/stress evolution and mechanism exploration in graphene electrodes during electrochemical process. Carbon, 144, 342–350.
Liu, D., Wang, Y., Xie, Y., He, L., Chen, J., Wu, K., Xu, R., & Gao, Y. (2013). On the stress characteristics of graphite anode in commercial pouch lithium-ion battery. Journal of Power Sources, 232, 29–33.
Jung, H. S., Gerasopoulos, K., Talin, A. A., & Ghodssi, R. (2017). A platform for in situ Raman and stress characterizations of V2O5 cathode using MEMS device. Electrochimica Acta, 242, 227–239.
Jung, H. S., Gerasopoulos, K., Talin, A. A., & Ghodssi, R. (2017). In situ characterization of charge rate dependent stress and structure changes in V2O5 cathode prepared by atomic layer deposition. Journal of Power Sources, 340, 89–97.
Cortes, F. J. Q., Boebinger, M. G., Xu, M., Ulvestad, A., & McDowell, M. T. (2018). Operando synchrotron measurement of strain evolution in individual alloying anode particles within lithium batteries. ACS Energy Letters, 3(2), 349–355.
Ali, I., Tippabhotla, S. K., Radchenko, I., Al-Obeidi, A., Stan, C. V., Tamura, N., & Budiman, A. (2018). Probing stress states in silicon nanowires during electrochemical lithiation using in situ synchrotron X-ray microdiffraction. Frontiers in Energy Research, 6, 1.
Tardif, S., Pavlenko, E., Quazuguel, L., Boniface, M., Maréchal, M., Micha, J., Gonon, L., Mareau, V. H., Gebel, G., Bayle-Guillemaud, P., Rieutord, F., & Lyonnard, S. (2017). Operando Raman spectroscopy and synchrotron X-ray diffraction of lithiation/delithiation in silicon nanoparticle anodes. ACS Nano, 11(11), 11306–11316.
Malavé, V., Berger, J., Zhu, H., & Kee, R. J. (2014). A computational model of the mechanical behavior within reconstructed LixCoO2 Li-ion battery cathode particles. Electrochimica Acta, 130, 707–717.
Wu, L., Wen, Y., & Zhang, J. (2016). Three-dimensional finite element study on Li diffusion induced stress in FIB-SEM reconstructed LiCoO2 half cell. Electrochimica Acta, 222, 814–820.
Wu, L., Xiao, X., Wen, Y., & Zhang, J. (2016). Three-dimensional finite element study on stress generation in synchrotron X-ray tomography reconstructed nickel-manganese-cobalt based half cell. Journal of Power Sources, 336, 8–18.
Han, S., Park, J., Lu, W., & Sastry, A. M. (2013). Numerical study of grain boundary effect on Li+ effective diffusivity and intercalation-induced stresses in Li-ion battery active materials. Journal of Power Sources, 240, 155–167.
Wu, W., Xiao, X., Wang, M., & Huang, X. (2014). A microstructural resolved model for the stress analysis of lithium-ion batteries. Journal of the Electrochemical Society, 161(5), A803–A813.
Yang, L., Chen, H., Song, W., & Fang, D. (2018). In situ optical observations and simulations on defect induced failure of silicon island anodes. Journal of Power Sources, 405, 101–105.
Xia, X., Han, J., Zhang, Q., Xiang, Y., & Hu, X. (2022). Real-time mechanical and thermal monitoring of lithium batteries with PVDF-TrFE thin films integrated within the battery. Sensors and Actuators A-Physical, 338, 113484.
Lee, C. H., Han, S. W., Lewis, J. S., Shetty, P. P., Yeh, D., Liu, Y., Klein, E. J., Lee, H., & McDowell, M. T. (2021). Stack pressure measurements to probe the evolution of the lithium–solid-state electrolyte interface. ACS Energy Letters, 6(9), 3261–3269.
Tanaka, S. (2019). Solid state reactions and sintering. In J. Hojo (Ed.), Materials chemistry of ceramics. Singapore: Springer.
Cronau, M., Szabo, M., König, C., Wassermann, T. N., & Roling, B. (2021). How to measure a reliable ionic conductivity? The stack pressure dilemma of microcrystalline sulfide-based solid electrolytes. ACS Energy Letters, 6(9), 3072–3077.
Palomares, V., & Rojo, T. (2012). Synthesis processes for Li-ion battery electrodes—From solid state reaction to solvothermal self-assembly methods. InTech EBooks.
Flores-González, N. A., Minafra, N., Dewald, G., Reardon, H., Smith, R. J. E., Adams, S., Zeier, W. G., & Gregory, D. H. (2021). Mechanochemical synthesis and structure of lithium tetrahaloaluminates, LiAlX4 (X = Cl, Br, I): A family of Li-ion conducting ternary halides. ACS Materials Letters, 3(5), 652–657.
Strauss, F., Zinkevich, T., Indris, S., & Brezesinski, T. (2020). Li7GeS5Br—An argyrodite Li-ion conductor prepared by mechanochemical synthesis. Inorganic Chemistry, 59(17), 12954–12959.
Kim, H., Kim, Y., Kim, D., Sohn, H. Y., & Kang, T. W. (2001). Mechanochemical synthesis and electrochemical characteristics of Mg2Sn as an anode material for Li-ion batteries. Solid State Ionics, 144(1–2), 41–49.
Kosova, N. V., Uvarov, N. F., Devyatkina, E. T., & Avvakumov, E. G. (2000). Mechanochemical synthesis of LiMn2O4 cathode material for lithium batteries. Solid State Ionics, 135(1–4), 107–114.
Rojac, T., & Kosec, M. (2010). Mechanochemical synthesis of complex ceramic oxides. Elsevier BV.
Boulineau, S., Courty, M., Tarascon, J., & Viallet, V. (2012). Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. In Solid state ionics. Elsevier BV.
West, A. R. (2022). Solid state chemistry and its applications. Wiley.
Hoff, L. C., Scheld, W. S., Uhlenbuck, S., Stollenwerk, J., Vedder, C., & Lobe, S. (2022). Laser sintering of ceramic-based solid-state battery materials. In Laser-based micro- and nanoprocessing XVI.
Laser sintering of ceramic-based solid-state battery materials. (2021). In L. C. Hoff, W. S. Scheld, C. Vedder, J. Stollenwerk, S. Lobe, & S. Uhlenbruck (Eds.), Lasers in Manufacturing Conference 2021. Wissenschaftliche Gesellschaft Lasertechnik.
Acord, K. A., Dupuy, A. D., Wang, X., Vyatskikh, A., Donaldson, O. K., Rupert, T. J., Wu, J. T., Chen, Q., & Schoenung, J. M. (2021). Microstructure, mechanical properties, and ionic conductivity of a solid-state electrolyte prepared using binderless laser powder bed fusion. Journal of Materials Research, 36(22), 4565–4577.
Acord, K. A., Dupuy, A. D., Bertoli, U. S., Zheng, B., West, W. N., Chen, Q., Shapiro, A. A., & Schoenung, J. M. (2021). Morphology, microstructure, and phase states in selective laser sintered lithium ion battery cathodes. Journal of Materials Processing Technology, 288, 116827.
Ramos, E. C. T., Browar, A. E. M., Roehling, J. D., & Ye, J. (2022). CO2 laser sintering of garnet-type solid-state electrolytes. ACS Energy Letters, 7(10), 3392–3400.
Rao, K. J., Vaidhyanathan, B., Ganguli, M., & Ramakrishnan, P. A. (1999). Synthesis of inorganic solids using microwaves. Chemistry of Materials, 11(4), 882–895.
Subramanian, V., Chen, C., Chou, H., & Fey, G. (2001). Microwave-assisted solid-state synthesis of LiCoO2 and its electrochemical properties as a cathode material for lithium batteries. Journal of Materials Chemistry, 11(12), 3348–3353.
Qiao, Y., Hu, X., Liu, Y., & Huang, Y. (2012). Li4Ti5O12 nanocrystallites for high-rate lithium-ion batteries synthesized by a rapid microwave-assisted solid-state process. Electrochimica Acta, 63, 118–123.
Corr, S. A., Amores, M., Ashton, T. E., Baker, P., & Cussen, E. J. (2016). Fast microwave-assisted synthesis of Li-stuffed garnets and insights into Li diffusion from muon spin spectroscopy. Journal of Materials Chemistry A Materials for Energy and Sustainability, 4(5), 1729–1736.
Liu, S., Yan, P., Li, H., Zhang, X., & Sun, W. (2020). One-step microwave synthesis of micro/nanoscale LiFePO4/graphene cathode with high performance for lithium-ion batteries. Frontiers in Chemistry, 8, 104.
Liu, J., Li, X., Yang, J., Geng, D., Li, Y., Wang, D., Li, R., Sun, X., Cai, M., & Verbrugge, M. W. (2012). Microwave-assisted hydrothermal synthesis of nanostructured spinel Li4Ti5O12 as anode materials for lithium ion batteries. Electrochimica Acta, 63, 100–104.
Chou, S. L., Wang, J. Z., Liu, H. K., & Dou, S. X. (2011). Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery. Journal of Physical Chemistry C, 115, 16220–16227.
Lee, W. S., & Lee, J. (2014). Novel synthesis of high performance anode materials for lithium-ion batteries (LIBs). Journal of Materials Chemistry A Materials for Energy and Sustainability, 2(6), 1589–1626.
Schmalzried, H. (1995). Chemical kinetics of solids. VCH.
Delaizir, G., Viallet, V., Aboulaich, A., Bouchet, R., Tortet, L., Seznec, V., Morcrette, M., Tarascon, J., Rozier, P., & Dollé, M. (2012). The stone age revisited: Building a monolithic inorganic lithium-ion battery. Advanced Functional Materials, 22(10), 2140–2147.
Liu, H., Wu, Y., Rahm, E., Holze, R., & Wu, H. (2004). Cathode materials for lithium ion batteries prepared by sol–gel methods. Journal of Solid State Electrochemistry, 8(7), 450–466.
Klein, L. (1988). Sol–gel technology. Park Ridge: Noyes.
Brinker, C. J., & Scherer, G. W. (1990). Sol–gel science. Academic Press.
Danks, A. E., Hall, S. R., & Schnepp, Z. (2016). The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Materials Horizons, 3(2), 91–112.
Ling, M., Zhu, X., Jiang, Y., & Zhu, J. (2016). Comparative study of solid-state reaction and sol-gel process for synthesis of Zr-doped Li0.5La0.5TiO3 solid electrolytes. Ionics, 22(11), 2151–2156.
Rajaeiyan, A., & Bagheri-Mohagheghi, M. (2013). Comparison of sol-gel and co-precipitation methods on the structural properties and phase transformation of γ and α-Al2O3 nanoparticles. Advances in Manufacturing, 1(2), 176–182.
Suvaci, E., & Özel, E. (2021). Hydrothermal synthesis (pp. 59–68). Elsevier.
Yan, H., Zhu, Z., Zhang, D., Li, W., & Qilu, A. M. (2012). A new hydrothermal synthesis of spherical Li4Ti5O12 anode material for lithium-ion secondary batteries. Journal of Power Sources, 219, 45–51.
Lin, Y.-S., & Duh, J.-G. (2011). Facile synthesis of mesoporous lithium titanate spheres for high rate lithium-ion batteries. Journal of Power Sources, 196, 10698–10703.
Zakharova, G. S., Thauer, E., Wegener, S. A., Nölke, J. H., Zhu, Q. M., & Klingeler, R. (2019). Hydrothermal microwave-assisted synthesis of Li3VO4 as an anode for lithium-ion battery. Journal of Solid State Electrochemistry, 23(7), 2205–2212.
Dong, H., & Koenig, G. (2020). A review on synthesis and engineering of crystal precursors produced via coprecipitation for multicomponent lithium-ion battery cathode materials. CrystEngComm, 22, 1514–1530.
Robinson, J. P., & Koenig, G. M. (2015). Tuning solution chemistry for morphology control of lithium-ion battery precursor particles. Powder Technology, 284, 225–230.
Kim, G.-H., Myung, S.-T., Bang, H. J., Prakash, J., & Sun, Y.-K. (2004). Synthesis and electrochemical properties of Li [Ni1/3Co1/3Mn (1/3− x) Mg x] O 2− y F y via coprecipitation. Electrochemical and Solid-State Letters, 7, A477.
Tahir, M., Rafique, M., Rafique, M., Nawaz, T., Rizwan, M., & Tanveer, M. (2020). Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals (pp. 119–138). Elsevier.
Bhawani, S., Khan, A., & Jawaid, M. (2020). Smart polymer nanocomposites. Woodhead Publishing.
Jung, D. S., Ko, Y. N., Kang, Y. C., & Park, S. B. (2014). Recent progress in electrode materials produced by spray pyrolysis for next-generation lithium ion batteries. Advanced Powder Technology, 25(1), 18–31.
Chen, G., & Wang, W. (2007). Role of freeze drying in nanotechnology. Drying Technology, 25(1), 29–35.
Wang, W., Wang, T., Xuecheng, F., Zhang, C., Hu, J., Chen, H. S., Fang, Z., Yan, J., & Liu, B. (2019). Freeze-drying-assisted synthesis of mesoporous CoMoO4 nanosheets as anode electrode material for enhanced lithium batteries. Chemical Research in Chinese Universities, 35(2), 261–270.
Surace, Y., Simões, M., Karvonen, L., Yoon, S., Pokrant, S., & Weidenkaff, A. (2015). Freeze drying synthesis of Li3MnO4 cathode material for Li-ion batteries: A physico-electrochemical study. Journal of Alloys and Compounds, 644, 297–303.
Blin, J., Stébé, M., & Lebeau, B. (2016). Hybrid/porous materials obtained from nano-emulsions. Current Opinion in Colloid & Interface Science, 25, 75–82.
Hu, J., Sun, C., Gillette, E., Gui, Z., Wang, Y., & Lee, S. (2016). Dual-template ordered mesoporous carbon/Fe2O3 nanowires as lithium-ion battery anodes. Nanoscale, 8, 12958–12969.
Wei, D., Cotton, D., & Ryhänen, T. (2012). All-solid-state textile batteries made from nano-emulsion conducting polymer inks for wearable electronics. Nanomaterials, 2, 268–274.
Guo, B., Wang, X., Fulvio, P., Chi, M., Mahurin, S., Sun, X., & Dai, S. (2011). Soft-templated mesoporous carbon–carbon nanotube composites for high performance lithium-ion batteries. Advanced Materials, 23, 4661–4666.
Kim, H., & Cho, J. (2008). Hard templating synthesis of mesoporous and nanowire SnO2 lithium battery anode materials. Journal of Materials Chemistry, 18, 771.
Park, G. T., Yoon, J., Park, E., Park, S. B., Kim, H., Kim, K. H., Jin, X., Shin, T. J., Kim, H., Yoon, W., & Kim, J. (2015). In operando monitoring of the pore dynamics in ordered mesoporous electrode materials by small angle X-ray scattering. ACS Nano, 9(5), 5470–5477.
Lin, Z., Yue, W., Huang, D., Hu, J., Zhang, X., Yuan, Z., & Yang, X. (2012). Pore length control of mesoporous Co3O4and its influence on the capacity of porous electrodes for lithium-ion batteries. RSC Advances, 2(5), 1794–1797.
Zhang, R., Li, N., Cheng, X., Yin, Y., Zhang, Q., & Guo, Y. (2017). Advanced micro/nanostructures for lithium metal anodes. Advanced Science, 4, 1600445.
Zhu, J., Ding, Y., Ma, Z., Tang, W., Chen, X., & Lu, Y. (2022). Recent progress on nanostructured transition metal oxides as anode materials for lithium-ion batteries. Journal of Electronic Materials, 51(7), 3391–3417.
Cui, Y., Liu, Y., Liang, Z., Lee, H., Sun, J., Wang, H., Yan, K., Xie, J., & Cui, Y. (2016). Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nature Nanotechnology, 11(7), 626–632.
Lu, L., Ge, J., Yang, J., Chen, S., Yao, H., & Zhou, F. (2016). Free-standing copper nanowire network current collector for improving lithium anode performance. Nano Letters, 16(7), 4431–4437.
Cheng, X., Hou, T., Zhang, R., Peng, H., Zhao, C., Huang, J., & Zhang, Q. (2016). Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Advanced Materials, 28(15), 2888–2895.
Reynolds, C., Slater, P., Hare, S., Simmons, M., & Kendrick, E. (2021). A review of metrology in lithium-ion electrode coating processes. Materials & Design, 209, 109971.
Schmitt, M., Scharfer, P., & Schabel, W. (2014). Slot die coating of lithium-ion battery electrodes: Investigations on edge effect issues for stripe and pattern coatings. Journal of Coatings Technology and Research, 11(1), 57–63.
Li, X., & Wang, C. (2013). Engineering nanostructured anodes via electrostatic spray deposition for high performance lithium ion battery application. Journal of Materials Chemistry A Materials for Energy and Sustainability, 1(2), 165–182.
Lee, S. Y., Huang, C. M., Johnston, C. I., & Grant, P. S. (2018). Spray printing and optimization of anodes and cathodes for high performance Li-Ion batteries. Electrochimica Acta, 292, 546–557.
Amrollahi, P., Krasinski, J. S., Vaidyanathan, R., Tayebi, L., & Vashaee, D. (2015). Electrophoretic deposition (EPD): Fundamentals and applications from nano- to micro-scale structures (pp. 1–27). Springer.
Ferrari, B., Moreno, R., Hernán, L., Melero, M., Morales, J., & Caballero, A. C. (2007). EPD of thick films for their application in lithium batteries. Journal of the European Ceramic Society, 27(13–15), 3823–3827.
Izumi, A., Sanada, M., Furuichi, K., Teraki, K., Matsuda, T., Hiramatsu, K., Munakata, H., & Kanamura, K. (2012). Development of high capacity lithium-ion battery applying three-dimensionally patterned electrode. Electrochimica Acta, 79, 218–222.
Maurel, A., Grugeon, S., Fleutot, B., Courty, M., Prashantha, K., Tortajada, H., Armand, M., Panier, S., & Dupont, L. (2019). Three-dimensional printing of a LiFePO4/graphite battery cell via fused deposition modeling. Scientific Reports, 9(1), 18031.
Gastol, D., Capener, M., Reynolds, C. J., Constable, C., & Kendrick, E. (2021). Microstructural design of printed graphite electrodes for lithium-ion batteries. Materials & Design, 205, 109720.
Bockholt, H., Indrikova, M., Netz, A., Golks, F., & Kwade, A. (2016). The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties. Journal of Power Sources, 325, 140–151.
Liu, D., Chen, L.-C., Liu, T.-J., Fan, T., Tsou, E.-Y., & Tiu, C. (2014). An effective mixing for lithium ion battery slurries. Advances in Chemical Engineering and Science, 4, 515–528.
Ludwig, B., Liu, J., Chen, I.-M., Liu, Y., Shou, W., Wang, Y., & Pan, A. H. (2017). Understanding interfacial-energy-driven dry powder mixing for solvent-free additive manufacturing of li-ion battery electrodes. Advanced Materials Interfaces, 4, 1700570.
Monnier, H., Wilhelm, A. M., & Delmas, H. (1999). The influence of ultrasound on micromixing in a semi-batch reactor. Chemical Engineering Science, 54, 2953–2961.
Kustersa, K. A., Pratsinisa, S. E., Thomab, S. G., & Smith, D. M. (1994). Energy—Size reduction laws for ultrasonic fragmentation. Powder Technology, 80, 253–263.
Kraytsberg, A., & Ein-Eli, Y. (2016). Conveying advanced Li-ion battery materials into practice the impact of electrode slurry preparation skills. Advanced Energy Materials, 6, 1600655.
Gutoff, E. B., & Cohen, E. D. (2006). Coating and drying defects (2nd ed.). Hoboken: Wiley.
Zhao, R., Liu, J., & Gu, J. (2015). The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery. Applied Energy, 139, 220–229.
Du, Z., Rollag, K., Li, J. C., An, S., Wood, M., Sheng, Y. P., Mukherjee, P. K., Daniel, C., & Wood, D. (2017). Enabling aqueous processing for crack-free thick electrodes. Journal of Power Sources, 354, 200–206.
Lin, Y., Liu, T., & Hwang, S. (2005). Minimum wet thickness for double-layer slide-slot coating of poly(vinyl-alcohol) solutions. Polymer Engineering and Science, 45(12), 1590–1599.
Chang, Y., Chang, H., Lin, C., Liu, T., & Wu, P. (2007). Three minimum wet thickness regions of slot die coating. Journal of Colloid and Interface Science, 308(1), 222–230.
Chang, H., Chang, Y., Lin, C., & Liu, T. (2007). Comparison of vertical and horizontal slot die coatings. Polymer Engineering and Science, 47(11), 1927–1936.
Kwon, N. H., Yin, H., Vavrova, T., Lim, J. H. W., Steiner, U., Grobéty, B., & Fromm, K. M. (2017). Nanoparticle shapes of LiMnPO4, Li+ diffusion orientation and diffusion coefficients for high volumetric energy Li+ ion cathodes. Journal of Power Sources, 342, 231–240.
J. V. Koleske, (1995) Paint and Coating Testing Manual: Fourteenth Edition of the Gardner-Sward Handbook.
Kuhn, H. A., & Ferguson, B. L. (1983). Pseudo-isostatic powder metallurgy densification process. Society of Automotive Engineers. SAE Technical Papers.
Appetecchi, G. B., Hassoun, J., Scrosati, B., Croce, F., Cassel, F., & Salomon, M. (2003). Hot-pressed, solvent-free, nanocomposite, PEO-based electrolyte membranes. Journal of Power Sources, 124(1), 246–253.
Sakuda, A., Hayashi, A., & Tatsumisago, M. (2017). Recent progress on interface formation in all-solid-state batteries. Current Opinion in Electrochemistry, 6(1), 108–114.
Jung, Y. S., Oh, D. K., Nam, Y., & Park, K. H. (2015). Issues and challenges for bulk-type all-solid-state rechargeable lithium batteries using sulfide solid electrolytes. Israel Journal of Chemistry, 55(5), 472–485.
Kuang, X., Carotenuto, G., & Nicolais, L. (1997). A review of ceramic sintering and suggestions on reducing sintering temperatures. Advanced Performance Materials, 4, 257–274.
Yang, L., et al. (2020). Rapid sintering method for highly conductive Li7La3Zr2O12 ceramic electrolyte. Ceramics International, 46(8A), 10917–10924.
Berbano, S. S., Guo, J., Guo, H., Lanagan, M. T., & Randall, C. A. (2017). Cold sintering process of Li 1.5 Al 0.5 Ge 1.5 (PO 4) 3 solid electrolyte. Journal of the American Ceramic Society, 100(5), 2123–2135.
Nakaya, H., Iwasaki, M., & Randall, C. A. (2020). Thermal-assisted cold sintering study of a lithium electrolyte: Li13.9Sr0.1Zn(GeO4)4. Journal of Electroceramics, 44(1-2), 16–22. https://doi.org/10.1007/s10832-019-00196-1
Seo, J. Y., Verlinde, K., Rajagopalan, R., Gomez, E. D., Mallouk, T. E., & Randall, C. A. (2019). Cold sintering process for fabrication of a high volumetric capacity Li4Ti5O12 anode. Materials Science and Engineering: B, 250, 114435.
Liu, Y., Sun, Q., Wang, D., Adair, K. R., Liang, J., & Sun, X. (2018). Development of the cold sintering process and its application in solid-state lithium batteries. Journal of Power Sources, 393, 193–203.
Boaretto, N., Garbayo, I., Valiyaveettil-SobhanRaj, S., Quintela, A., Li, C., Casas-Cabanas, M., & Aguesse, F. (2021). Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. Journal of Power Sources, 502, 229919.
Bates, J. B. (2000). Thin-film lithium and lithium-ion batteries. Solid State Ionics, 135(1–4), 33–45.
Nagao, M., Hayashi, A., & Tatsumisago, M. (2012). Bulk-type lithium metal secondary battery with indium thin layer at interface between Li electrode and Li2S-P2S5 solid electrolyte. Electrochemistry, 80(10), 734–736.
Fu, K., Gong, Y., Liu, B., Zhu, Y., Xu, S., Yao, Y., Li, W., Wang, C., Lacey, S. D., Dai, J., Chen, Y., Mo, Y., Wachsman, E. D., & Hu, L. (2017). Toward garnet electrolyte–based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Science Advances, 3(4), e1601659.
Fu, K., Gong, Y., Fu, Z., Xie, H., Yao, Y., Liu, B., Carter, M., Wachsman, E. D., & Hu, L. (2017). Transient behavior of the metal interface in lithium metal-garnet batteries. Angewandte Chemie, 56(47), 14942–14947.
Tsai, C., Roddatis, V., Chandran, C. V., Ma, Q., Uhlenbruck, S., Bram, M., Heitjans, P., & Guillon, O. (2016). Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Applied Materials & Interfaces, 8(16), 10617–10626.
Wakasugi, J., Munakata, H., & Kanamura, K. (2017). Effect of gold layer on interface resistance between lithium metal anode and Li6.25Al0.25La3Zr2O12 solid electrolyte. Journal of the Electrochemical Society, 164(6), A1022–A1025.
Wang, L., Zhang, L., Wang, Q., Li, W., Wu, B., Jia, W., Wang, Y., Li, J., & Li, H. (2018). Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Materials, 10, 16–23.
Liu, K., Zhang, R., Wu, M. C., Jiang, H., & Zhao, T. (2019). Ultra-stable lithium plating/stripping in garnet-based lithium-metal batteries enabled by a SnO2 nanolayer. Journal of Power Sources, 433, 226691.
Rawlence, M., Filippin, A. N., Wäckerlin, A., Lin, T., Cuervo-Reyes, E., Remhof, A., Battaglia, C., Rupp, J. L. M., & Buecheler, S. (2018). Effect of gallium substitution on lithium-ion conductivity and phase evolution in sputtered Li7–3xGaxLa3Zr2O12 thin films. ACS Applied Materials & Interfaces, 10(16), 13720–13728.
Yu, X., Bates, J. H. T., Jellison, G. E., Jr., & Hart, F. X. (1997). A stable thin-film lithium electrolyte: Lithium phosphorus oxynitride. Journal of the Electrochemical Society, 144(2), 524–532.
Greer, J. C. (2014). History and current status of commercial pulsed laser deposition equipment. Journal of Physics D, 47(3), 034005.
Julien, C. M., & Mauger, A. (2019). Pulsed laser deposited films for microbatteries. Coatings, 9(6), 386.
Aguesse, F., Roddatis, V., Roqueta, J., García, P. A., Pergolesi, D., Santiso, J., & Kilner, J. A. (2015). Microstructure and ionic conductivity of LLTO thin films: Influence of different substrates and excess lithium in the target. Solid State Ionics, 272, 1–8.
Pfenninger, R., Struzik, M., Garbayo, I., Stilp, E., & Rupp, J. L. M. (2019). A low ride on processing temperature for fast lithium conduction in garnet solid-state battery films. Nature Energy, 4(6), 475–483.
Knoops, H. H., Donders, M. M., De Mcm Richard Sanden, V., Notten, P. P., & Kessels, W. E. (2012). Atomic layer deposition for nanostructured Li-ion batteries. Journal of Vacuum Science & Technology, 30(1), 010801.
Wang, G., Lu, C., Zhang, X., Wan, B., Liu, H., Xia, M., Gou, H., Xin, G., Lian, J., & Zhang, Y. (2017). Toward ultrafast lithium ion capacitors: A novel atomic layer deposition seeded preparation of Li4Ti5O12/graphene anode. Nano Energy, 36, 46–57.
Nisula, M., Shindo, Y., Koga, H., & Karppinen, M. (2015). Atomic layer deposition of lithium phosphorus oxynitride. Chemistry of Materials, 27(20), 6987–6993.
Kozen, A. C., Pearse, A. J., Lin, C., Noked, M., & Rubloff, G. W. (2015). Atomic layer deposition of the solid electrolyte LiPON. Chemistry of Materials, 27(15), 5324–5331.
Kazyak, E., Chen, K., Wood, K. A., Davis, A. M., Thompson, T., Bielinski, A. R., Sanchez, A. A., Wang, X., Wang, C., Sakamoto, J., & Dasgupta, N. P. (2017). Atomic layer deposition of the solid electrolyte garnet Li7La3Zr2O12. Chemistry of Materials, 29(8), 3785–3792.
Liu, J., Banis, M. N., Sun, Q., Sun, X., Li, R., & Sham, T. (2014). Rational design of atomic-layer-deposited LiFePO4as a high-performance cathode for lithium-ion batteries. Advanced Materials, 26(37), 6472–6477.
Donders, M. M., Arnoldbik, W., Knoops, H. H., Kessels, W. E., & Notten, P. P. (2013). Atomic layer deposition of LiCoO2 thin-film electrodes for all-solid-state Li-ion micro-batteries. Journal of the Electrochemical Society, 160(5), A3066–A3071.
Lu, W., Liang, L., Sun, X., Sun, X., Wu, C., Hou, L., Sun, J., & Yuan, C. (2017). Recent progresses and development of advanced atomic layer deposition towards high-performance Li-ion batteries. Nanomaterials, 7(10), 325.
Liu, J., Zhu, H., & Shiraz, M. H. A. (2018). Toward 3D solid-state batteries via atomic layer deposition approach. Frontiers in Energy Research, 6, 10.
Lalau, C. C., & Low, C. K. (2019). Electrophoretic deposition for lithium-ion battery electrode manufacture. Batteries & Supercaps, 2(6), 551–559.
Yang, Y., Chen, D., Liu, B., & Zhao, J. (2015). Binder-free Si nanoparticle electrode with 3D porous structure prepared by electrophoretic deposition for lithium-ion batteries. ACS Applied Materials & Interfaces, 7(14), 7497–7504.
Azuma, S., Aiyama, K., Kawamura, G., Muto, H., Mizushima, T., Uchikoshi, T., & Matsuda, A. (2017). Colloidal processing of Li2S-P2S5 films fabricated via electrophoretic deposition methods and their characterization as a solid electrolyte for all solid state lithium ion batteries. Journal of the Ceramic Society of Japan, 125(4), 287–292.
Seino, Y., Ota, T., Takada, K., Hayashi, A., & Tatsumisago, M. (2014). A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy and Environmental Science, 7(2), 627–631.
Lavoie, P.-A., Dube, J., Gagnon, Y., & Laliberte, R. (2010). EP1568090B1—Co-extrusion manufacturing process of thin film electrochemical cell for lithium polymer batteries.
Lavoie, P.-A., Laliberté, R., Dubé, J., & Gagnon, Y. (2010). Co-extrusion manufacturing process of thin film electrochemical cell for lithium polymer batteries and apparatus Therefor, no. US7700019B2.
Baudry, P., Lascaud, S., Majastre, H., & Bloch, D. (1997). Lithium polymer battery development for electric vehicle application. Journal of Power Sources, 68(2), 432–435.
Zhai, Y., Liu, H., Li, L., Yu, J., & Ding, B. (2019). Electrospun nanofibers for lithium-ion batteries (pp. 671–694). Elsevier.
Kim, J. H., Choi, S. H., Jo, S. J., Lee, W. Y., & Kim, B. Y. (2004). Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochimica Acta, 50(1), 69–75.
Fu, K., Gong, Y., Dai, J., Gong, A. G. W., Han, X., Yao, Y., Wang, C., Wang, Y., Chen, Y., Yan, C., Li, Y., Wachsman, E. D., & Hu, L. (2016). Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proceedings of the National Academy of Sciences of the United States of America, 113(26), 7094–7099.
Liu, W., Liu, N., Sun, J., Hsu, P., Li, Y., Lee, H., & Cui, Y. (2015). Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Letters, 15(4), 2740–2745.
Bin Mamtaz, M. R., Wang, Z., Belotti, A., Quattrocchi, E., Yu, J., Liu, J., & Ciucci, F. (2021). Enhancing Ni exsolution by nonmetal B-site substituents (Si and P) in SrTiO3-based solid oxide fuel cell anodes. ACS Energy & Fuels, 35(18), 15084–15093.
Meyer, C., Bockholt, H., Haselrieder, W., & Kwade. (2017). A. Characterization of the calendering process for compaction of electrodes for lithium-ion batteries. Journal of Materials Processing Technology, 249, 172–178.
Günther, T., Schreiner, D., Metkar, A., Meyer, C., Kwade, A., & Reinhart, G. (2019). Classification of calendering-induced electrode defects and their influence on subsequent processes of lithium-ion battery production. Energy Technology, 8, 1900026.
Sangrós, G. C., Finke, B., Schilde, C., Froböse, L., & Kwade, A. (2019). Numerical simulation of the behavior of lithium-ion battery electrodes during the calendaring process via the discrete element method. Powder Technology, 349, 1–11.
Johnson, L., & Johnson, D. (2017). EP3168914A1—Solid-state batteries and methods of fabrication thereof.
Kurfer, J., Westermeier, M., Tammer, C., & Reinhart, G. (2012). Production of large-area lithium-ion cells—Preconditioning, cell stacking and quality assurance. CIRP Annals-Manufacturing Technology, 61, 1–4.
Patwa, R., Herfurth, H., Heinemann, S., Mazumder, J., & Lee, D. (2012). Investigation of different laser cutting strategies for sizing of Li-ion battery electrodes. In International Congress on Applications of Lasers & Electro-Optics, Vol. 908.
Stich, M., Pandey, N., & Bund, A. (2017). Drying and moisture resorption behaviour of various electrode materials and separators for lithium-ion batteries. Journal of Power Sources, 364, 84–91.
Huttner, F., Haselrieder, W., & Kwade, A. (2019). The influence of different post-drying procedures on remaining water content and physical and electrochemical properties of lithium-ion batteries. Energy Technol., 8, 1900245.
Reinhart, G., Zeilinger, T., Kurfer, J., Westermeier, M., Thiemann, C., Glonegger, M., Wunderer, M., Tammer, C., Schweier, M., & Heinz, M. (2013). Research and demonstration center for the production of largearea lithium-ion cells. In G. Schuh, R. Neugebauer, & E. Uhlmann (Eds.), Future trends in production engineering (pp. 3–12). Springer.
Kariatsumari, K. (2010). Toyota announces 4-layer all-solid-state battery. http://techon.nikkeibp.co.jp/english/NEWS_EN/20101122/187553
Satou, A. (2017). US20170263981—Bipolar laminated all-solid-state lithium-ion rechargeable battery and method for manufacturing same.
Yang, N., Zhang, X., Shang, B., & Li, G. (2016). Unbalanced discharging and aging due to temperature differences among the cells in a lithium-ion battery pack with parallel combination. Journal of Power Sources, 306, 733–741.
Tsuruta, K., Dermer, M. E., & Dhiman, R. (2020). WO2020096973A1—Cell with a tabless electrode.
Bhattacharya, S., & Alpas, A. T. (2012). Micromechanisms of solid electrolyte interphase formation on electrochemically cycled graphite electrodes in lithium-ion cells. Carbon, 50, 5359–5371.
Mckinley, J. P., Sellers, S. A., & Colclazier, K. R. (2010). Battery formation and charging system and method. 12/604,234. US patent number: US20100164437A1.
Lv, H., Huang, X., & Liu, Y. (2020). Analysis on pulse charging–discharging strategies for improving capacity retention rates of lithium-ion batteries. Ionics, 26, 1749–1770.
Grape, U. (2015). SEEO Final Technical Report, Recovery Act—Solid state batteries for grid-scale energy storage.
Xu, L., Lu, Y., Zhao, C., Yuan, H., Zhu, G., Hou, L., Zhang, Q., & Huang, J. (2020). Toward the scale-up of solid-state lithium metal batteries: The gaps between lab-level cells and practical large-format batteries. Advanced Energy Materials, 11, 2002360.
McCloskey, B. D. (2015). Attainable gravimetric and volumetric energy density of Li–S and Li ion battery cells with solid separator-protected Li metal anodes. Journal of Physical Chemistry Letters, 6(22), 4581–4588.
Liu, J., Bao, Z., Cui, Y., Dufek, E. J., Goodenough, J. B., Khalifah, P., Li, Q., Liaw, B. Y., Liu, P., Manthiram, A., Meng, Y. S., Subramanian, V. R., Toney, M. F., Viswanathan, V. V., Whittingham, M. S., Xiao, J., Xu, W., Yang, J., Yang, X. Q., & Zhang, J. G. (2019). Pathways for practical high-energy long-cycling lithium metal batteries. Nature Energy, 4, 180.
Wang, C., Yu, R., Duan, H., Lu, Q., Li, Q., Adair, K. R., Bao, D., Liu, Y., Yang, R., Wang, J., Zhao, S., Huang, H., & Sun, X. (2021). Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Letters, 7(1), 410–416.
Zhang, K., Wu, F., Wang, X., Weng, S., Yang, X., Zhao, H., Guo, R., Sun, Y., Zhao, W., Song, T., Wang, X., Bai, Y., & Wu, C. (2022). 8.5 µm-thick flexible-rigid hybrid solid electrolyte/lithium integration for air-stable and interface-compatible all-solid-state lithium metal batteries. Advanced Energy Materials, 12(24), 2200368.
Bao, C., Zheng, C., Wu, M., Zhang, Y., Jin, J., Chen, H., & Wen, Z. (2023). 12 µm-thick sintered garnet ceramic skeleton enabling high-energy-density solid-state lithium metal batteries. Advanced Energy Materials, 2023, 2204028.
Acknowledgements
The authors would like to acknowledge the support from Natural Sciences and Engineering Research Council (NSERC) of Canada and Alberta Innovates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is an invited paper (Invited Review).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bin Mamtaz, M., Michaud, X., Jo, H. et al. Stress and Manufacturability in Solid-State Lithium-Ion Batteries. Int. J. of Precis. Eng. and Manuf.-Green Tech. 10, 1093–1137 (2023). https://doi.org/10.1007/s40684-023-00519-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40684-023-00519-2