Abstract
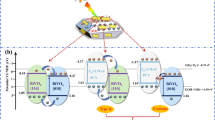
Green technology such as harvesting solar light is a promising source for future energy supplies without generating any harmful environmental pollutions. We report a straightforward approach for improving the light harvesting properties of BiVO4 photoanodes in order to enhance their photoelectrochemical performance. A facile sol–gel spin coating method was used to prepare the samples. The g-C3N4 powder was incorporated into the BiVO4 precursor solution before being spin-coated onto fluorine-doped tin oxide glass. After annealing, a thin film of g-C3N4 modified BiVO4 (CN-BiVO4) was formed. A WO3 film was coated onto CN-BiVO4 (CN-BiVO4/WO3) to form a Z-scheme heterojunction and enhance the photoelectrochemical performance. The CN-BiVO4 and CN-BiVO4/WO3 photoanodes outperformed unmodified photoanodes in terms of light harvesting and photoelectrochemical performance. The CN-BiVO4/WO3 had the highest light absorbance and photocurrent density (538 µA·cm− 2 at 1.23 VRHE) and significantly enhanced resilience and stability (with a 35% decay for 1600 s) compared with unmodified electrodes. At 390 nm, the incident photon-to-current conversion efficiency was 14.8%, and the applied bias photon-to-current conversion efficiency was approximately 0.12% at 0.88 VRHE. These findings reveal that the non-crystalline phases of CN-BiVO4 grains help absorb and trap a broader range of wavelengths, thus improving photoelectrochemical performance.








Similar content being viewed by others
References
Midilli, A., Ay, M., Dincer, I., & Rosen, M. A. (2005). On hydrogen and hydrogen energy strategies: I: Current status and needs. Renewable and Sustainable Energy Reviews, 9(3), 255–271. https://doi.org/10.1016/j.rser.2004.05.003
Bak, D., & Kim, J. H. (2017). Facile fabrication of pseudo-microspherical ZnO/CdS core-shell photocatalysts for solar hydrogen production by water splitting. Ceramics International, 43(16), 13493–13499. https://doi.org/10.1016/j.ceramint.2017.07.054
Momirlan, M., & Veziroglu, T. N. (2002). Current status of hydrogen energy. Renewable and Sustainable Energy Reviews, 6(1–2), 141–179. https://doi.org/10.1016/S1364-0321(02)00004-7
Khaselev, O., & Turner, J. A. (1998). A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting. Science, 280(5362), 425–427. https://doi.org/10.1126/science.280.5362.425
Vaghasiya, J., Nandakumar, D. K., Yaoxin, Z., & Tan, S. C. (2018). Low toxicity environmentally friendly single component aqueous organic ionic conductors for high efficiency photoelectrochemical solar cells. Journal of Materials Chemistry A, 6(3), 1009–1016. https://doi.org/10.1039/c7ta09557k
Vijselaar, W. J. C., Perez-Rodriguez, P., Westerik, P. J., Tiggelaar, R. M., Smets, A. H. M., Gardeniers, H., & Huskens, J. (2019). A Stand-Alone Si-Based Porous Photoelectrochemical Cell. Advanced Energy Materials, 9(19), https://doi.org/10.1002/aenm.201803548
Luo, B., Liu, J., Guo, H., Liu, X., Song, R., Shen, K., Wang, Z. M., Jing, D., Selopal, G. S., & Rosei, F. (2021). High efficiency photoelectrochemical hydrogen generation using eco-friendly Cu doped Zn-In-Se colloidal quantum dots. Nano Energy, 88. https://doi.org/10.1016/j.nanoen.2021.106220
Kahng, S., Yoo, H., & Kim, J. H. (2020). Recent advances in earth-abundant photocatalyst materials for solar H2 production. Advanced Powder Technology, 31(1), 11–28. https://doi.org/10.1016/j.apt.2019.08.035
Walter, M. G., Warren, E. L., McKone, J. R., Boettcher, S. W., Mi, Q., Santori, E. A., & Lewis, N. S. (2010). Solar water splitting cells. Chemical Reviews, 110(11), 6446–6473. https://doi.org/10.1021/cr1002326
Jang, J. S., Kim, H. G., & Lee, J. S. (2012). Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catalysis Today, 185(1), 270–277. https://doi.org/10.1016/j.cattod.2011.07.008
FUJISHIMA, A., & HONDA, K. (1972). Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238(5358), 37–38. https://doi.org/10.1038/238037a0
Tahir, A. A., Wijayantha, U., Saremi-Yarahmadi, K. G., Maznar, S., M., & McKee, V. (2009). Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation. Chemistry of Materials, 21(16), 3763–3772. https://doi.org/10.1021/cm803510v
Hahn, N. T., Ye, H., Flaherty, D. W., Bard, A. J., & Mullins, C. B. (2010). Reactive ballistic deposition of α-Fe2O3 thin films for photoelectrochemical water oxidation. Acs Nano, 4(4), 1977–1986. https://doi.org/10.1021/nn100032y
Ng, C., Ng, Y. H., Iwase, A., & Amal, R. (2013). Influence of annealing temperature of WO3 in photoelectrochemical conversion and energy storage for water splitting. ACS Applied Materials and Interfaces, 5(11), 5269–5275. https://doi.org/10.1021/am401112q
Li, W., Da, P., Zhang, Y., Wang, Y., Lin, X., Gong, X., & Zheng, G. (2014). WO3 nanoflakes for enhanced photoelectrochemical conversion. Acs Nano, 8(11), 11770–11777. https://doi.org/10.1021/nn5053684
Kim, J. H., & Lee, J. S. (2019). Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Advanced Materials, 31(20), https://doi.org/10.1002/adma.201806938
Lee, D. K., & Choi, K. S. (2018). Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nature Energy, 3(1), 53–60. https://doi.org/10.1038/s41560-017-0057-0
Lu, X., Liu, Z., Li, J., Zhang, J., & Guo, Z. (2017). Novel framework g-C3N4 film as efficient photoanode for photoelectrochemical water splitting. Applied Catalysis B: Environmental, 209, 657–662. https://doi.org/10.1016/j.apcatb.2017.03.030
Babu, B., Shim, J., & Yoo, K. (2020). Efficient solar-light-driven photoelectrochemical water oxidation of one-step in-situ synthesized Co-doped g-C3N4 nanolayers. Ceramics International, 46(10), 16422–16430. https://doi.org/10.1016/j.ceramint.2020.03.203
Ma, Q. B., Hofmann, J. P., Litke, A., & Hensen, E. J. M. (2015). Cu2O photoelectrodes for solar water splitting: Tuning photoelectrochemical performance by controlled faceting. Solar Energy Materials and Solar Cells, 141, 178–186. https://doi.org/10.1016/j.solmat.2015.05.025
Chen, Y. C., Chen, Y. J., Popescu, R., Dong, P. H., Gerthsen, D., & Hsu, Y. K. (2019). Defect-Cluster-Boosted Solar Photoelectrochemical Water Splitting by n-Cu2O Thin Films Prepared Through Anisotropic Crystal Growth. ChemSusChem, 12(21), 4859–4865. https://doi.org/10.1002/cssc.201901798
Wolcott, A., Smith, W. A., Kuykendall, T. R., Zhao, Y., & Zhang, J. Z. (2009). Photoelectrochemical study of nanostructured ZnO thin films for hydrogen generation from water splitting. Advanced Functional Materials, 19(12), 1849–1856. https://doi.org/10.1002/adfm.200801363
Lv, R., Wang, T., Su, F., Zhang, P., Li, C., & Gong, J. (2014). Facile synthesis of ZnO nanopencil arrays for photoelectrochemical water splitting. Nano Energy, 7, 143–150. https://doi.org/10.1016/j.nanoen.2014.04.020
Stefik, M., Cornuz, M., Mathews, N., Hisatomi, T., Mhaisalkar, S., & Grätzel, M. (2012). Transparent, conducting Nb:SnO2 for host-guest photoelectrochemistry. Nano Letters, 12(10), 5431–5435. https://doi.org/10.1021/nl303101n
Radecka, M., Wnuk, A., Trenczek-Zajac, A., Schneider, K., & Zakrzewska, K. (2015). TiO2/SnO2 nanotubes for hydrogen generation by photoelectrochemical water splitting. International Journal of Hydrogen Energy, 40(1), 841–851. https://doi.org/10.1016/j.ijhydene.2014.09.154
Li, Y., Takata, T., Cha, D., Takanabe, K., Minegishi, T., Kubota, J., & Domen, K. (2013). Vertically aligned Ta3N5 nanorod arrays for solar-driven photoelectrochemical water splitting. Advanced Materials, 25(1), 125–131. https://doi.org/10.1002/adma.201202582
Wang, L., Zhou, X., Nguyen, N. T., Hwang, I., & Schmuki, P. (2016). Strongly Enhanced Water Splitting Performance of Ta3N5 Nanotube Photoanodes with Subnitrides. Advanced Materials, 28(12), 2432–2438. https://doi.org/10.1002/adma.201505312
Yoo, H., & Kim, J. H. (2021). Photoactive TiO2/CuxO composite films for photocatalytic degradation of methylene blue pollutant molecules. Advanced Powder Technology, 32(4), 1287–1293. https://doi.org/10.1016/j.apt.2021.02.031
Choi, T., Kim, J. S., & Kim, J. H. (2016). Influence of alkoxide structures on formation of TiO2/WO3 heterojunctions for photocatalytic decomposition of organic compounds. Advanced Powder Technology, 27(5), 2061–2065. https://doi.org/10.1016/j.apt.2016.07.016
Kshirsagar, A. S., & Khanna, P. K. (2019). CuSbSe2/TiO2: Novel type-II heterojunction nano-photocatalyst. Materials Chemistry Frontiers, 3(3), 437–449. https://doi.org/10.1039/c8qm00537k
Yoo, H., Kahng, S., & Kim, J. H. (2020). Z-scheme assisted ZnO/Cu2O-CuO photocatalysts to increase photoactive electrons in hydrogen evolution by water splitting. Solar Energy Materials and Solar Cells, 204. https://doi.org/10.1016/j.solmat.2019.110211
Choi, T., Kim, J. S., & Kim, J. H. (2016). Transparent nitrogen doped TiO2/WO3 composite films for self-cleaning glass applications with improved photodegradation activity. Advanced Powder Technology, 27(2), 347–353. https://doi.org/10.1016/j.apt.2016.01.005
Liu, G., Karuturi, S. K., Chen, H., Wang, D., Ager, J. W., Simonov, A. N., & Tricoli, A. (2020). Enhancement of the photoelectrochemical water splitting by perovskite BiFeO3 via interfacial engineering. Solar Energy, 202, 198–203. https://doi.org/10.1016/j.solener.2020.03.117
Gelderman, K., Lee, L., & Donne, S. W. (2007). Flat-band potential of a semiconductor: using the Mott–Schottky equation. Journal of chemical education, 84(4), 685. https://doi.org/10.1021/ed084p685
Wang, Z., Nguyen, T. D., Yeo, L. P., Tan, C. K., Gan, L., & Tok, A. I. Y. (2020). Periodic FTO IOs/CdS NRs/CdSe Clusters with Superior Light Scattering Ability for Improved Photoelectrochemical Performance. Small (Weinheim An Der Bergstrasse, Germany), 16(6), https://doi.org/10.1002/smll.201905826
Madhusudan, P., Ran, J., Zhang, J., Yu, J., & Liu, G. (2011). Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Applied Catalysis B: Environmental, 110, 286–295. https://doi.org/10.1016/j.apcatb.2011.09.014
Zhang, Z., Wang, M., Cui, W., & Sui, H. (2017). Synthesis and characterization of a core-shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation. RSC Advances, 7(14), 8167–8177. https://doi.org/10.1039/c6ra27766g
Chen, P. W., Chang, C., te, Ko, T. F., Hsu, S. C., Li, K. D., & Wu, J. Y. (2020). Fast response of complementary electrochromic device based on WO3/NiO electrodes. Scientific Reports, 10(1), https://doi.org/10.1038/s41598-020-65191-x
Chatchai, P., Murakami, Y., Kishioka, S., ya, Nosaka, A. Y., & Nosaka, Y. (2009). Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation. Electrochimica Acta, 54(3), 1147–1152. https://doi.org/10.1016/j.electacta.2008.08.058
Zhang, L., Chen, D., & Jiao, X. (2006). Monoclinic structured BiVO4 nanosheets: Hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties. Journal of Physical Chemistry B, 110(6), 2668–2673. https://doi.org/10.1021/jp056367d
Bai, S., Zhang, K., Wang, L., Sun, J., Luo, R., Li, D., & Chen, A. (2014). Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. Journal of Materials Chemistry A, 2(21), 7927–7934. https://doi.org/10.1039/c4ta00053f
Kahng, S., & Kim, J. H. (2021). Optimal oxidation of CuxZn1–xS photocatalysts for enhanced solar H2 production by efficient charge separations. Ceramics International, 47(2), 2848–2856. https://doi.org/10.1016/j.ceramint.2020.09.139
Kang, H., & Kim, J. H. (2017). Utilization of a ZnS(en)0.5 photocatalyst hybridized with a CdS component for solar energy conversion to hydrogen. Advanced Powder Technology, 28(9), 2438–2444. https://doi.org/10.1016/j.apt.2017.07.001
El-Hagary, M., Shaaban, E. R., Moustafa, S. H., & Gad, G. M. A. (2019). The particle size-dependent optical band gap and magnetic properties of Fe-doped CeO2 nanoparticles. Solid State Sciences, 91, 15–22. https://doi.org/10.1016/j.solidstatesciences.2019.03.005
Sarkar, S., & Chattopadhyay, K. K. (2012). Size-dependent optical and dielectric properties of BiVO4 nanocrystals. Physica E: Low-dimensional Systems and Nanostructures, 44(7–8), 1742–1746. https://doi.org/10.1016/j.physe.2011.11.019
Yoo, H., Lee, D., & Kim, J. H. (2021). High performance of TiO2/CuxO photoelectrodes for regenerative solar energy storage in a vanadium photoelectrochemical cell. Green Energy and Environment, 7(4), 704–711. https://doi.org/10.1016/j.gee.2020.11.012
Meyer, J., Hamwi, S., Kröger, M., Kowalsky, W., Riedl, T., & Kahn, A. (2012). Transition metal oxides for organic electronics: energetics, device physics and applications. Advanced materials, 24(40), 5408–5427. https://doi.org/10.1002/adma.201201630
Tran, V. H., Park, H., Eom, S. H., Yoon, S. C., & Lee, S. H. (2018). Modified SnO2 with alkali carbonates as robust electron-transport layers for inverted organic solar cells. ACS omega, 3(12), 18398–18410. https://doi.org/10.1021/acsomega.8b02773
Xu, Q., Zhang, L., Cheng, B., Fan, J., & Yu, J. (2020). S-scheme heterojunction photocatalyst. Chem, 6(7), 1543–1559. https://doi.org/10.1016/j.chempr.2020.06.010
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2021R1F1A1059276). This work was also partially supported by Korea Environment Industry & Technology Institute (KEITI) through Post Plastic, a specialized program of the Graduate School funded by Korea Ministry of Environment (MOE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sang, P., Kim, J.H. Role of g-C3N4 in Fabrication of BiVO4/WO3 Z-scheme Heterojunction for high Photoelectrochemical Performances with Enhanced Light Harvesting. Int. J. of Precis. Eng. and Manuf.-Green Tech. 10, 1015–1026 (2023). https://doi.org/10.1007/s40684-022-00478-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40684-022-00478-0