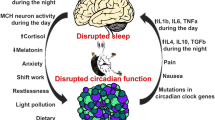
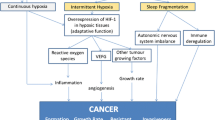
Abstract
Sleep-disordered breathing (SDB) has been linked to wide range of adverse physiologic and pathologic features, from cognitive impairments to increased risk of cancer. In this current review, we will discuss some of the emerging evidence connecting sleep disruption with cancer, by exploring different models of sleep disorders and how they appear to affect cancer. Then, we will summarize our current understanding of the underlying mechanisms, and finally, we will discuss the impact of sleep on one of the frequent and advanced cancer therapies, namely bone marrow transplantation (BMT). Experiments in animal models provide supportive findings, whereby each of the major components of SDB has begun to unravel their potential and complex effects on cancer biology, most likely via alterations in oxidative stress, angiogenesis, immune function, and sympathetic outflow. Future research is undoubtedly needed to better understand the pathophysiologic mechanisms of SDB-related effects on cancer in general, and more specifically on cancer prevention, treatment (chemotherapy, radiation, BMT), and outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190–4. doi:10.1164/rccm.201201-0130OC.
Marshall NS, Wong KKH, Cullen SRJ, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–62. doi:10.5664/jcsm.3600.
Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99–105. doi:10.1164/rccm.201209-1671OC.
Owens RL, Gold KA, Gozal D, et al. Sleep and breathing … and cancer? Cancer Prev Res (Phila). 2016;9(11):821–7. doi:10.1158/1940-6207.CAPR-16-0092.
Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep. 2016;39(8):1493–500. doi:10.5665/sleep.6004.
Dal Molin M, Brant A, Blackford AL, et al. Obstructive sleep apnea and pathological characteristics of resected pancreatic ductal adenocarcinoma. Real FX, ed. PLoS One. 2016;11(10):e0164195. doi:10.1371/journal.pone.0164195.
Lee S, Kim BG, Kim JW, et al. Obstructive sleep apnea is associated with an increased risk of colorectal neoplasia. Gastrointest Endosc. 2016;85(3):568–573.e1. doi:10.1016/j.gie.2016.07.061.
Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273–84. doi:10.1016/j.smrv.2012.08.003.
Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16(11):1381–7. doi:10.1016/j.sleep.2015.02.543.
Rao D, Yu H, Bai Y, Zheng X, Xie L. Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. Onco Targets Ther. 2015;8:2817–26. doi:10.2147/OTT.S89769.
Wang X, Ji A, Zhu Y, et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015;6(28):25046–60. doi:10.18632/oncotarget.4502.
• Gozal D, Farré R, Nieto FJ. Putative links between sleep apnea and cancer: from hypotheses to evolving evidence. Chest. 2015;148(5):1140–7. doi:10.1378/chest.15-0634. This paper opened many research areas in the field of sleep and cancer by proposing different pathophysiological aspects of this relationship.
Martínez-García MÁ, Campos-Rodríguez F, Almendros I, Farré R. Relationship between sleep apnea and cancer. Arch Bronconeumol. 2015;51(9):456–61. doi:10.1016/j.arbres.2015.02.002.
Cao J, Feng J, Li L, Chen B. Obstructive sleep apnea promotes cancer development and progression: a concise review. Sleep Breath. 2015;19(2):453–7. doi:10.1007/s11325-015-1126-x.
Abrams B. Cancer and sleep apnea—the hypoxia connection. Med Hypotheses. 2007;68(1):232. doi:10.1016/j.mehy.2006.06.037.
Rofstad EK, Gaustad J-V, Egeland TAM, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127(7):1535–46. doi:10.1002/ijc.25176.
Almendros I, Montserrat JM, Ramírez J, Torres M, Duran-Cantolla J, Navajas DFR. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39(1):215–7. doi:10.1183/09031936.00021111.
Almendros I, Montserrat JM, Torres M, Bonsignore MR, Chimenti L, Navajas DFR. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13(10):1254–60. doi:10.1016/j.sleep.2012.08.012.
•• Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, Schoenfelt KS, Carreras A, Hakim F, Zhang SX, Farré RGD. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189(5):593–601. doi:10.1164/rccm.201310-1830OC. This well-controlled study was the first to explore the impact of intermittent hypoxia on cancer progression from its immunological aspect.
Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186(3):303–7. doi:10.1016/j.resp.2013.03.001.
Eubank T, Sherwani S, Peters S, Gross A, Evans RMU. Intermittent hypoxia augments melanoma tumor metastases in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2013;187:A2302. http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A2302 . Accessed 3 Dec 2016
Nair D, Zhang SXL, Ramesh V, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305–12. doi:10.1164/rccm.201107-1173OC.
Ramesh V, Nair D, Zhang SXL, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91.
•• Hakim F, Wang Y, Zhang SXL, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–37. doi:10.1158/0008-5472.CAN-13-3014. With this paper the authors showed for the first time that Tumor Associated Macrophages via a TLR 4 pathway, are involved in the effect of fragmented sleep on tumor growth and its aggressive behavior.
Zheng J, Almendros I, Wang Y, et al. Reduced NADPH oxidase type 2 activity mediates sleep fragmentation-induced effects on TC1 tumors in mice. Oncoimmunology. 2015;4(2):e976057. doi:10.4161/2162402X.2014.976057.
Khalyfa A, Almendros I, Gileles-Hillel A, et al. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget. 2016; doi:10.18632/oncotarget.10578.
Almendros I, Khalyfa A, Trzepizur W, et al. Tumor cell malignant properties are enhanced by circulating exosomes in sleep apnea. Chest. 2016;150(5):1030–41. doi:10.1016/j.chest.2016.08.1438.
Akbarpour M, Khalyfa A, Qiao Z, et al. Altered CD8+ T-Cell lymphocyte function and TC1 cell stemness contribute to enhanced malignant tumor properties in murine models of sleep apnea. Sleep. 2016.
Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain Behav Immun. 2012;26(4):672–9. doi:10.1016/j.bbi.2012.03.002.
Maragno-Correa JMR, Patti CL, Zanin KA, et al. Sleep deprivation increases mortality in female mice bearing Ehrlich ascitic tumor. Neuroimmunomodulation. 2013;20(3):134–40. doi:10.1159/000346201.
De Lorenzo BHP, de Oliveira ML, Greco CR, Suchecki D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology. 2015;57:134–43. doi:10.1016/j.psyneuen.2015.04.006.
Coelho M, Soares-Silva C, Brandão D, Marino F, Cosentino M, Ribeiro L. β-adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017;143(2):275–91. doi:10.1007/s00432-016-2278-1.
He R-H, He Y-J, Tang Y-J, Zhou H-H, McLeod HL, Liu J. The potential anticancer effect of beta-blockers and the genetic variations involved in the interindividual difference. Pharmacogenomics. 2016;17(1):74–9. doi:10.2217/pgs.15.152.
Childers WK, Hollenbeak CS, Cheriyath P. β-blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin Breast Cancer. 2015;15(6):426–31. doi:10.1016/j.clbc.2015.07.001.
Colucci R, Moretti S. The role of stress and beta-adrenergic system in melanoma: current knowledge and possible therapeutic options. J Cancer Res Clin Oncol. 2016;142(5):1021–9. doi:10.1007/s00432-015-2078-z.
Golombek DA, Casiraghi LP, Agostino PV, et al. The times they’re a-changing: effects of circadian desynchronization on physiology and disease. J Physiol Paris. 2013;107(4):310–22. doi:10.1016/j.jphysparis.2013.03.007.
Filipski E, Lévi F. Circadian disruption in experimental cancer processes. Integr Cancer Ther. 2009;8(4):298–302. doi:10.1177/1534735409352085.
Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8(4):337–46. http://www.ncbi.nlm.nih.gov/pubmed/20050373 . Accessed 3 Dec. 2016
Iwamoto A, Kawai M, Furuse M, Yasuo S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol Int. 2014;31(2):189–98. doi:10.3109/07420528.2013.837478.
Wood PA, Yang X, Hrushesky WJM. Clock genes and cancer. Integr Cancer Ther. 2009;8(4):303–8. doi:10.1177/1534735409355292.
Magnone MC, Langmesser S, Bezdek AC, Tallone T, Rusconi S, Albrecht U. The mammalian circadian clock gene per2 modulates cell death in response to oxidative stress. Front Neurol. 2014;5:289. doi:10.3389/fneur.2014.00289.
Mteyrek A, Filipski E, Guettier C, Okyar A, Lévi F. Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget. 2016; doi:10.18632/oncotarget.11037.
Papagiannakopoulos T, Bauer MR, Davidson SM, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 2016;24(2):324–31. doi:10.1016/j.cmet.2016.07.001.
Van Dycke KCG, Rodenburg W, van Oostrom CTM, et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr Biol. 2015;25(14):1932–7. doi:10.1016/j.cub.2015.06.012.
Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–12. doi:10.1038/nrc3610.
Poroyko VA, Carreras A, Khalyfa A, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. doi:10.1038/srep35405.
Moreno-Indias I, Torres M, Sanchez-Alcoholado L, et al. Normoxic recovery mimicking treatment of sleep apnea does not reverse intermittent hypoxia-induced bacterial dysbiosis and low-grade endotoxemia in mice. Sleep. 2016;39(10):1891–7. doi:10.5665/sleep.6176.
Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055–65. doi:10.1183/09031936.00184314.
Gozal D, Gileles-Hillel A, Cortese R, et al. Visceral white adipose tissue following chronic intermittent and sustained hypoxia in mice. Am J Respir Cell Mol Biol. 2017; doi:10.1165/rcmb.2016-0243OC.
Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell MOl Physiol. 2014;307(2):L129–40. doi:10.1152/ajplung.00089.2014.
Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275:2991–3002. doi:10.1111/j.1742-4658.2008.06454.x.
Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–60. doi:10.1038/emboj.2012.125.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–14. doi:10.1016/j.tips.2012.01.005.
Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor—HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–7. http://www.ncbi.nlm.nih.gov/pubmed/22300081
Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cance. 2007;121(11):2381–6. doi:10.1002/ijc.23192.
Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32(1):1–15. doi:10.1042/BSR20100136.
Ruffell B, Affara NICL. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–26. doi:10.1016/j.it.2011.12.001.Differential.
Gozal D, Almendros I, Hakim F. Sleep apnea awakens cancer: a unifying immunological hypothesis. Oncoimmunology. 2014;3:e28326. doi:10.4161/onci.28326.
Almendros I, Gileles-hillel A, Khalyfa A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett. 2015;361(2):233–9. doi:10.1016/j.canlet.2015.03.010.
Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol. 2012;3:7. doi:10.3389/fneur.2012.00007.
Cole SWSA. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201–6. doi:10.1158/1078-0432.CCR-11-0641.Molecular.
Almendros I, Khalyfa A, Gilelels-Hillel A, Qiao Z, Farre R, Gozal D. Intermittent hypoxia-induced adrenergic alterations modulate tumor proliferation in a mouse model of obstructive sleep apnea. Am J Respir Crit Care Med. 2015;191:A2699.
Méndez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16(4):235–42. doi:10.1097/MOH.0b013e32832bd0f5.
Ieyasu A, Tajima Y, Shimba S, Nakauchi H, Yamazaki S. Clock gene Bmal1 is dispensable for intrinsic properties of murine hematopoietic stem cells. J Negat Results Biomed. 2014;13:4. doi:10.1186/1477-5751-13-4.
Guariniello LD, Vicari P, Lee KS, de Oliveira AC, Tufik S. Bone marrow and peripheral white blood cells number is affected by sleep deprivation in a murine experimental model. J Cell Physiol. 2012;227(1):361–6. doi:10.1002/jcp.22743.
Lungato L, Nogueira-Pedro A, Carvalho Dias C, Paredes-Gamero EJ, Tufik S, D’Almeida V. Effects of sleep deprivation on mice bone marrow and spleen B lymphopoiesis. J Cell Physiol. 2016;231(6):1313–20. doi:10.1002/jcp.25231.
• Rolls A, Pang WW, Ibarra I, et al. Sleep disruption impairs haematopoietic stem cell transplantation in mice. Nat Commun. 2015;6:8516. doi:10.1038/ncomms9516. This is a well controlled study that explores not only the impact of sleep disorders on tumor growth, but also its impact on one of its most important treatments, bone marrow transplantation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Karin Yaacoby-Bianu and Fahed Hakim each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep and Cancer
Rights and permissions
About this article
Cite this article
Yaacoby-Bianu, K., Hakim, F. Sleep Disturbance and Cancer—Animal Models. Curr Sleep Medicine Rep 3, 31–37 (2017). https://doi.org/10.1007/s40675-017-0073-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-017-0073-4