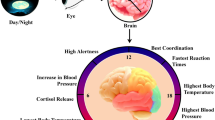
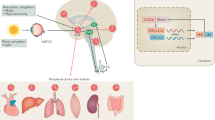
Abstract
Purpose of Review
A wide array of sleep and circadian deficits has been observed in patients with Alzheimer’s disease (AD). However, the vast majority of these studies have focused on later-stage AD and do not shed light on the possibility that circadian dysfunction contributes to AD pathogenesis. The goal of this review is to examine the evidence supporting or refuting the hypothesis that circadian dysfunction plays an important role in early AD pathogenesis or AD risk in humans.
Recent Findings
Few studies have addressed the role of the circadian system in very early AD or prior to AD diagnosis. AD appears to have a long presymptomatic phase during which pathology is present, but cognition remains normal. Studies evaluating circadian function in cognitively normal elderly or early-stage AD have thus far not incorporated AD biomarkers. Thus, the cause-and-effect relationship between circadian dysfunction and early-stage AD remains unclear.
Summary
Circadian dysfunction becomes apparent in AD as dementia progresses, but it is unknown at which point in the pathogenic process rhythms begin to deteriorate. Further, it is unknown if exposure to circadian disruption in middle age increases AD risk later in life. This review addresses gaps in current knowledge on this topic and proposes several critical directions for future research which might help to clarify the potential pathogenic role of circadian clock dysfunction in AD.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Alzheimer’s Association. Alzhiemer’s disease facts and figures. Alzheimers Dement. 2015;11:332–84.
Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry. 2001;158:704–11. doi:10.1176/appi.ajp.158.5.704.
Holth JK, Patel TK, Holtzman DM. Sleep in Alzheimer’s disease-beyond amyloid. Neurobiol Sleep Circad Rhythym. 2016; doi:10.1016/j.nbscr.2016.08.002.
Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9. doi:10.1038/nrneurol.2013.269.
Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi:10.1038/emm.2014.121.
Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2014;19:29–38.
McClung CR. Plant circadian rhythms. Plant Cell. 2006;18:792–803.
Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–64. doi:10.1038/nature11088.
Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62.
Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH. Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harb Perspect Biol. 2017;9:a027706. doi:10.1101/cshperspect.a027706.
Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–69. doi:10.1038/nrn3086.
Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010;22:362–72. doi:10.1111/j.1365-2826.2010.01956.x.
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi:10.1126/science.1238599.
Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–83. doi:10.1038/nn1419.
An S, Harang R, Meeker K, Granados-Fuentes D, Tsai CA, Mazuski C, et al. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci U S A. 2013;110:E4355–61. doi:10.1073/pnas.1307088110.
Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013;110:1097–106. doi:10.1152/jn.00114.2013.
Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–8.
Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–8. doi:10.1073/pnas.0902768106.
Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K, et al. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav Brain Res. 2016;308:222–35. doi:10.1016/j.bbr.2016.04.027.
Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri TD. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun. 2017;8:14336. doi:10.1038/ncomms14336.
Nakazato R, Hotta S, Yamada D, Kou M, Nakamura S, Takahata Y, et al. The intrinsic microglial clock system regulates interleukin-6 expression. Glia. 2017;65:198–208. doi:10.1002/glia.23087.
Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–9. doi:10.1016/j.bbi.2014.11.009.
Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, et al. Cross-talk among ROR alpha 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol. 1994;8:1253–61. doi:10.1210/mend.8.9.7838158.
Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006;2:e16. doi:10.1371/journal.pcbi.0020016.
Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–24. doi:10.1073/pnas.1408886111.
Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9. doi:10.1073/pnas.1114410109.
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi:10.1038/nature09253.
Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–7. doi:10.1161/CIRCULATIONAHA.108.827477.
Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, et al. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2016; doi:10.1096/fj.201601030R.
Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–8. doi:10.1126/science.1240636.
Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A. 2015;112:7231–6. doi:10.1073/pnas.1501327112.
Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–400. doi:10.1172/JCI70317.
Knutsson A, Kempe A. Shift work and diabetes—a systematic review. Chronobiol Int. 2014;31:1146–51. doi:10.3109/07420528.2014.957308.
Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62.
Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315:1726–34. doi:10.1001/jama.2016.4454.
Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210.
Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging. 1982;3:371–7.
Vitiello MV, Prinz PN. Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99.
Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–72.
Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991;14:478–85.
Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92.
Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16:765–71.
Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23.
Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–68. doi:10.1176/appi.ajgp.13.5.359.
Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717:154–9.
Prinz PN, Christie C, Smallwood R, Vitaliano P, Bokan J, Vitiello MV, et al. Circadian temperature variation in healthy aged and in Alzheimer’s disease. J Gerontol. 1984;39:30–5.
Weissova K, Bartos A, Sladek M, Novakova M, Sumova A. Moderate changes in the circadian system of Alzheimer’s disease patients detected in their home environment. PLoS One. 2016;11:e0146200. doi:10.1371/journal.pone.0146200.
Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127:1061–74. doi:10.1093/brain/awh129.
Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. 2005;1:155–63.
Mieda M, Okamoto H, Sakurai T. Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr Biol. 2016;26:2535–42. doi:10.1016/j.cub.2016.07.022.
• Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–22. doi:10.1002/ana.24432. Provides correlation between circadian rhythms and SCN VIP neuron count.
Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44.
Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–6.
Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–47. doi:10.1016/j.neurobiolaging.2006.06.002.
Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, et al. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–17. doi:10.1093/brain/awn049.
Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–6. doi:10.1096/fj.05-4446fje.
Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–21.
Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pevet P, Ravid D, et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res. 1990;528:170–4.
Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38:199–206.
Lim AS, Myers AJ, Yu L, Buchman AS, Duffy JF, De Jager PL, et al. Sex difference in daily rhythms of clock gene expression in the aged human cerebral cortex. J Biol Rhythm. 2013;28:117–29. doi:10.1177/0748730413478552.
Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:206–11. doi:10.1073/pnas.1508249112.
Cermakian N, Lamont EW, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer’s disease patients and control subjects. J Biol Rhythm. 2011;26:160–70. doi:10.1177/0748730410395732.
Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, et al. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Neurosci. 2014;17:377–82. doi:10.1038/nn.3651.
Lim AS, Srivastava GP, Yu L, Chibnik LB, Xu J, Buchman AS, et al. 24-hour rhythms of DNA methylation and their relation with rhythms of RNA expression in the human dorsolateral prefrontal cortex. PLoS Genet. 2014;10:e1004792. doi:10.1371/journal.pgen.1004792.
• Cronin P, McCarthy MJ, Lim AS, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2016; doi:10.1016/j.jalz.2016.10.003. First demonstration of altered Bmal1 promoter methylation in AD.
Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–42. doi:10.1212/wnl.0000000000001831.
Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013;1:65. doi:10.1186/2051-5960-1-65.
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 2016; doi:10.1111/bpa.12424.
Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi:10.1126/scitranslmed.3002369.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi:10.1002/ana.20009.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi:10.1016/j.jalz.2011.03.003.
Jack Jr CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi:10.1016/s1474-4422(09)70299-6.
Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–65. doi:10.1016/s1474-4422(13)70194-7.
Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi:10.1212/WNL.0b013e3182918ca6.
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi:10.1016/s1474-4422(13)70044-9.
Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–22. doi:10.1038/nature08538.
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi:10.1056/NEJMoa1202753.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi:10.1016/j.jalz.2011.03.008.
• Naismith SL, Hickie IB, Terpening Z, Rajaratnam SM, Hodges JR, Bolitho S, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38:857–66. doi:10.3233/jad-131217. This is one of the only papers examining sleep changes in mild cognitive impairment.
•• Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. doi:10.1001/jamaneurol.2013.2334. This is the only paper to date examining the relationship between CSF Aβ biomarker status and sleep parameters in cognitively normal people.
Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32.
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi:10.1001/jama.2015.4668.
Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36:1027–32. doi:10.5665/sleep.2802.
Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry. 2014;22:1262–71.
Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. doi:10.1001/jamaneurol.2013.4258.
Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–75.
• Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. Beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–7. doi:10.1038/nn.4035. Explores a unique potential mechanism linking Aβ pathology to SWS disruption.
Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi:10.5665/sleep.1732.
Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–8. doi:10.1038/88384.
Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66.
Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011;30:4642–51. doi:10.1038/emboj.2011.322.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi:10.1126/scitranslmed.3003748.
• Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi:10.1126/science.1241224. First description of a role for sleep in the regulation of the glymphatic clearance system.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309. doi:10.1172/JCI67677.
Acknowledgements
Erik S. Musiek is funded by NINDS K08 award K08NS079405 and an award from the Coins for Alzheimer’s Trust (CART).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Erik S. Musiek reports grants from NIH, Alzheimer’s Association, Coins for Alzheimer’s Trust, during the conduct of the study; personal fees from Eisai Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Circadian Rhythm Disorders
Rights and permissions
About this article
Cite this article
Musiek, E.S. Circadian Rhythms in AD Pathogenesis: a Critical Appraisal. Curr Sleep Medicine Rep 3, 85–92 (2017). https://doi.org/10.1007/s40675-017-0072-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-017-0072-5