Abstract
Adequate sleep is crucial for general health and wellbeing. Although the neuronal control of the reproductive axis and sleep-generating neurons share an anatomical location, little is known regarding the impact of sleep and circadian disruption on fertility in women. Animal models have established clear circadian control of the pre-ovulatory luteinizing hormone surge. Additionally, disruption of the circadian timing system by exposure to abnormal light-dark cycles or mutations of core clock genes results in diminished reproductive capacity in animals. Abnormalities in menstruation, fertility, and early pregnancy maintenance in female shift workers provide evidence for a role of circadian rhythms in the reproductive health of women. Reproductive hormones may modify sleep, and the relationship is bidirectional such that sleep disruption may alter the profile of reproductive hormone secretion. Therefore, sleep, apart from its circadian timing, may also have relevance in attaining pregnancy. Additionally, infertility is associated with psychological distress which may result in poor quality sleep. The interaction between psychological distress and disturbed sleep in reproduction has garnered minimal attention and may be a crucial factor to consider during the evaluation and treatment of infertility. This work reviews animal models and evidence in women that suggest a role for sleep and circadian rhythms in reproductive health and reveals areas that require future investigation.
Similar content being viewed by others
Introduction
Infertility is defined as the inability to conceive after 12 months of regular, unprotected intercourse in women less than 35 years of age or after 6 months in women over 35 years of age. The prevalence of infertility in the USA is estimated at 15 %, and 5 billion dollars are spent yearly on its evaluation and treatment [117].
The World Health Organization (WHO) found that among 8500 couples, infertility was attributed to solely female factors in 37 % [127]. Many female conditions cause or contribute to infertility. This manuscript appraises the available literature and describes potential links between sleep and circadian disruption and female infertility.
The ovulatory cycle is a critical factor in reproductive success. The hypothalamic-pituitary-gonadal (HPG) axis refers to the anatomical locations that mediate the ovulatory cycle, which is approximately 28 days in duration [88]. The hypothalamus controls the ovulatory cycle by secreting gonadotropin-releasing hormone (GnRH) that stimulates the pituitary to synthesize and release luteinizing hormone (LH) and follicle stimulating hormone (FSH) [88]. The ovulatory cycle is divided into the follicular phase, which begins the first day of menses and concludes the day prior to LH surge, and the luteal phase, which begins the day of the LH surge [88].
During the follicular phase, pulsatile GnRH secretion results in FSH production, which acts on the ovary to promote follicular development [88]. The developing ovarian follicle produces estradiol which exerts negative feedback on hypothalamus and pituitary, suppressing FSH and LH release [88]. Estradiol continues to rise during the late follicular phase and peaks the day before ovulation. Coinciding with this sustained peak, the feedback of estradiol switches from negative to positive and results in the LH surge [88]. The mechanism underlying the change from negative to positive feedback is not fully understood [26]. The LH surge is followed by release of the oocyte from the dominant ovarian follicle.
After ovulation, the ruptured ovarian follicle develops the corpus luteum, which produces both estradiol and progesterone during the luteal phase. The endometrium thickens in preparation to receive a fertilized oocyte. In the absence of implantation of a fertilized oocyte, the corpus luteum regresses in approximately 14 days, estradiol and progesterone decline, and menses occurs [88].
The most common cause of female infertility is ovulatory dysfunction (including that accompanying reproductive aging); however, anatomical abnormalities (particularly of the fallopian tubes or uterus), endometriosis, cervical factors, medical disorders, or lifestyle factors may also be responsible.
Biological Rationale that Circadian Rhythms Modify Female Fertility
Circadian rhythms are the near 24-h processes that allow an organism to coordinate appropriate physiological responses to the environmental light-dark changes associated with the rotation of the earth. The most obvious circadian rhythm is the sleep-wake cycle, which typically aligns to the external light-dark cycle. In normally entrained individuals, the circadian rhythm interacts with the homeostatic sleep drive in an opposing manner to provide consolidated wakefulness during the daytime hours and continuous sleep during the night [18].
Circadian rhythms are inherent to the organism and generated by a genetic transcriptional-translational feedback loop comprised of the following core clock genes: Bmal1, Clock, Per, and Cry [59]. Together, Clock and Bmal1 activate transcription of Per and Cry which form heterodimers in cytoplasm and translocate back into the nucleus to inhibit their own transcription by repressing the Clock: Bmal1 complex [59]. The entire cycle takes approximately 24 h to complete [59].
Circadian rhythms persist apart from sleep-wake behavior and external time cues. However, circadian rhythms are modifiable, particularly by light that entrains the central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus [92]. Circadian rhythmicity is evident in the biological processes of multiple organ systems and circadian oscillation of clock genes has been demonstrated in tissues throughout the body [92].
Ample evidence derived largely from animal models demonstrates circadian regulation of critical reproductive events.
Animal Models
Ovulatory Cycle
The infradian (>24 h) pre-ovulatory LH surge also demonstrates a circadian rhythm. The circadian regulation of the LH surge is crucial to ensure that ovulation and the window for oocyte fertilization overlap with the time when mating can feasibly occur.
The first evidence of circadian control of ovulation was more than 50 years ago by Drs. Everett and Sawyer who found the LH surge was always witnessed in the afternoon in female rats. When barbiturates (to provide global neuronal blockade) were administered at the expected time of the LH surge, the LH surge was suppressed and did not recur until 24-h later, long after the effect of barbiturate had waned. These findings suggested a neuronal control of the LH surge that cycled with a 24-h period [37].
Since that time, the circadian control of the pre-ovulatory LH surge by the SCN has been well documented in rodent models and is timed such that ovulation is closely tied to the start of the animals’ active period (for a comprehensive review, see [129]). Notably, the SCN triggers the LH surge only in the context of estrogen levels exceeding a critical threshold aligning with the change from negative to positive feedback of estrogen on gonadotropin release [101, 129]. The finding that exogenous administration of estradiol results in a daily, similarly timed LH surge in rats and mice highlights the requirement of sufficient estrogen levels in the circadian modulation of the LH surge [27, 63, 64].
The SCN controls the pre-ovulatory LH surge through efferent signals to GnRH neurons located in the preoptic area. The SCN sends projections to GnRH neurons directly via vasoactive intestinal peptide (VIP). However, the primary, daily signal from the SCN to the reproductive system is indirect and achieved by arginine vasopressin (AVP) signaling to kisspeptin neurons of the anteroventral periventricular (AVPV) nuclei. Kisspeptin is a potent stimulator of GnRH release. Kisspeptin signaling is thought to be essential for circadian input to the reproductive axis and coordination of the shift from negative to positive feedback of estrogen on GnRH neurons (for a comprehensive review, see [17, 101, 129]).
Both lesions of the SCN [20, 126] and mutations of core clock genes result in disruption of the LH surge and estrus cycle [16, 28, 33, 74, 86, 87]. Additionally, externally induced disruption achieved by a shift in the normal light-dark cycle results in desynchrony between the new external photoperiod and the LH surge and ovulation [72, 75, 76]. Re-entrainment to the new light-dark period is similar to that of locomotor activity with faster re-alignment to a delay as opposed to advance of the light-dark schedule.
In addition to the circadian control of ovulation, subsequent reproductive events demonstrate 24-h timing and core components of the molecular clock have been found in peripheral reproductive organs. Disruption of circadian timing either by genetic manipulation or alteration to the light-dark cycle results in impaired fertility in animal models.
Ovarian Function
Rhythmic oscillation of clock gene expression has been demonstrated in the ovary, steroid producing granulosa and theca cells lining the follicle, and the oocyte [95]. Apart from the circadian control of the LH surge, the ovarian response to gonadotropins demonstrates 24-h timing as exogenous LH can only trigger ovulation at a certain time of day in the rodent model [95]. Therefore, the peripheral ovarian clock may mediate the circadian variation in sensitivity to gonadotropins and, as evident by animal models with knockout of core clock genes, impact oocyte maturation and steroidogenesis [95].
Embryo Implantation
Clock gene expression has been documented in the uterus [50, 78, 87, 120], oviduct [50, 52], and pre-implantation embryo [50]. Therefore, circadian oscillation in the peripheral reproductive organs may play a role in early embryo development and implantation.
In support of this hypothesis, female mice with knock out of Bmal1, which results in loss of the circadian rhythm of behavior and gene expression both in the SCN and periphery [21], have failure of embryo implantation [16, 87]. This finding may be secondary to impaired steroidogenesis; however, implantation is only partially restored by exogenous progesterone supplementation [87]. Additionally, reduced implantation sites are observed when mice are subjected to a non-24 h day beginning 2–6 weeks prior to mating; however, both maternal and paternal contributions may be responsible as males and females were exposed to the abnormal light-dark cycle [35].
Early Gestation
Clock gene oscillation persists in the uterus and placenta during pregnancy [3]. Therefore, circadian input from maternal tissues may have implications for early gestation.
In fact, mutation of the gene Clock, which alters circadian period length and impairs sustained circadian rhythmicity [123], resulted in increased fetal resorption and non-productive labor compared to wild type animals [74]. More recently, abnormal Clock expression was found in spontaneously aborted mice fetuses [67, 68]. Middle aged, but not young, Per mutant animals demonstrated frequent post-implantation pregnancy loss [86].
Additionally, when external shifts of the light-dark cycle to mimic jet lag or shift work were initiated after confirmed copulation, a marked reduction in successful pregnancies was observed. Productive matings were lowest in females undergoing repetitive 6-h phase advances of the light-dark cycle (22 %) but also significantly decreased in the phase delay group (50 %) compared to control light-dark cycle (>90 %) [110]. Fetal loss was suspected early in pregnancy; however, the exact reproductive mechanism impacted by the light-dark shift cannot be determined [110]. Additionally, mice subjected to non-24 h days beginning on gestation day zero were found to have more resorbed or dead embryos [35].
Human
Although much less evidence is available, the circadian system may also influence timing of reproductive processes in women. Notably, the central circadian pacemaker, the SCN, contains estrogen and progesterone receptors [62].
Ovulatory Cycle
In addition to the infradian (>24 h) ovulatory cycle every 28 days, women display 24-h rhythmicity of the pre-ovulatory LH surge that typically occurs in the early morning hours [23, 53, 54].
However, the above studies are limited as the effect of sleep was not uncoupled from diurnal variation. Therefore, the rhythm of LH surge cannot be confirmed as circadian. In fact, the single study that used constant routine to isolate circadian rhythms from sleep found no circadian rhythm of LH secretion [55]. Additionally, because ovulation occurs approximately 36 h after the LH surge and normal sperm are capable of fertilizing an ovum in the female reproductive tract for up to 5 days, the current literature suggests the fertile window in women is on the order of days [128], as opposed to the tighter coupling seen in rodents [17]. Therefore, the presence and relevance of circadian timing of the pre-ovulatory LH surge in women remains uncertain. As in other mammals, kisspeptin is a potent stimulator of GnRH secretion from the human hypothalamus and ongoing work aims to determine the role of kisspeptin in the timing of the LH surge and switch from negative to positive estrogenic feedback on gonadotropin release [102].
Interestingly, bright light therapy may influence the ovulatory cycle. Bright light exposure delivered during various circadian times in women undergoing a 90-min ultra-short sleep-wake cycle protocol increased LH secretion from baseline [61]. Women were thought to be in the follicular stage of their menstrual cycle but this was not verified hormonally. In the same study, but with use of a different protocol, both morning bright light and (to a lesser extent) evening bright light increased LH secretion in the follicular and luteal stages of the menstrual cycle [61]. Another investigation that exposed women in the follicular stage of the menstrual cycle to a 45-min light pulse soon after awakening found increased levels of prolactin, FSH, and LH [30]. Ovarian follicle size and number of ovulatory cycles also increased [30]. Unfortunately, these studies were not designed to determine whether the impact of bright light on the ovulatory cycle is mediated by circadian phase shift, melatonin suppression, or changes in sleep.
On the other side of this bidirectional relationship, hormonal changes corresponding to the different phases of the ovulatory cycle may exert influence on circadian rhythms. Core body temperature displays a circadian rhythm with the nadir occurring typically 2–3 h before habitual sleep offset. During the progesterone predominate luteal phase, overall temperature is higher and the nocturnal decline in core body temperature is blunted [8, 94, 97]. Circadian rhythms of cortisol and thyroid stimulating hormone are also diminished during the luteal phase [99]. In conjunction with decreased amplitude of the circadian rhythm, naturally cycling reproductive age women demonstrate an increase in subjective daytime sleepiness and increased slow wave sleep during daytime naps [99]. Additionally, stage REM sleep is reduced in association with increased core body temperature during the luteal phase [8, 94, 97].
In the late follicular phase of the menstrual cycle, when estrogen levels are highest, core body temperature is decreased [8, 97]. Despite the changes in absolute temperature and amplitude of the circadian rhythm of core body temperature, the phase of the temperature rhythm appears stable over the course of the menstrual cycle [8, 10, 97].
Steroid Hormones
Sex steroids display diurnal variation. In premenopausal women with normal menstrual cycles, estradiol and progesterone peak in the early morning after awakening along with cortisol; however, unlike the well-established 24-h cortisol rhythm that persists when separated from sleep and waking behavior, true circadian variation has not been confirmed by protocols removing the influence of circadian zeitgebers ([1, 12, 22].)
Ovarian Function
Although evidence of molecular clock machinery has been well described in the non-human mammalian ovary [95], circadian influence on human ovarian function is unknown. However, a single study demonstrated diurnal variation in anti-Müllerian hormone (AMH), a marker frequently used to assess ovarian reserve in the clinical setting. AMH secretion demonstrated a small peak at 08:00 a.m. [22]. Further, the change in AMH over 24 h co-varied with LH secretion [22]. This interesting finding warrants further investigation given the circadian variation of ovarian responsiveness to gonadotropins seen in animals [95].
Embryo Implantation and Early Gestation
Implantation of the embryo in the uterus is a highly coordinated event requiring a receptive endometrium, good quality embryo, and appropriate interaction between the two [100]. Peripheral clock function in the uterus could contribute to these critical reproductive events. Bmal1 and Per2 expression have been demonstrated in both the non-pregnant and gravid uterine myometrium in women [15]. More relevant for fertility is the recent finding that Per2 is also rhythmically expressed in the human endometrial stromal cells of the uterus and its expression is downregulated during decidualization [77]. When this downregulation is premature, the decidual response is disorganized. In fact, endometrial biopsies during the midluteal phase from patients with recurrent miscarriages demonstrated an inverse correlation between Per2 transcript levels and number of failed pregnancies [77]. These findings suggest that intact circadian timing of reproductive events subsequent to ovulation may be required for successful conception.
An investigation of single-nucleotide polymorphisms in circadian clock genes may strengthen this hypothesis as polymorphisms in Bmal1 increased risk of miscarriages and polymorphisms in the Clock homolog NPAS2 were associated with decreased miscarriage [60].
Melatonin
The rhythm of endogenous melatonin secretion from the pineal gland is an output of the circadian clock and suppressed by light. Endogenous melatonin also provides feedback to the circadian timing system to reinforce entrainment to the 24-h day and appropriately timed exogenous melatonin can be used to produce shifts in circadian phase [65]. In addition to its function at the level of the central clock, melatonin receptors are located in numerous organs [48, 103] and apart from its action on receptors, melatonin functions as a free radical scavenger [42, 89].
The relevance of melatonin for reproduction was first understood in animals that breed seasonally. The duration of melatonin secretion provides information regarding day length to the reproductive axis [90]. Consequently, reproductive functions are optimized such that mating and conception are appropriately timed for parturition to occur during a season where conditions can sustain offspring [90].
Melatonin levels are particularly high in human ovarian follicular fluid and reflect both pineal and ovarian production. The role of melatonin in the ovary may be protection of the oocyte from oxidative stress, particularly during ovulation. In fact, lower follicular melatonin levels were associated with higher levels of reactive oxidative species and decreased oocyte quality in infertile women [113].
Knowledge of melatonin’s ability to reduce oxidative stress in the ovary has prompted the investigation of exogenous melatonin as a protective agent during in vitro fertilization (IVF) [90]. Melatonin supplementation (3 mg with duration of treatment lasting from 2 weeks to 3 months) has resulted in improvement of IVF outcomes including number of oocytes retrieved [36], oocyte maturation and quality [36, 91, 121], fertilization rate [82, 113, 121], and embryo quality [36, 82, 91, 121]. Improvements in IVF outcomes with melatonin supplementation have also been noted specifically in women with polycystic ovary syndrome (PCOS) [83]. Because of these promising early findings, a double-blind randomized placebo-controlled dose-response trial has been devised [39].
However, whether circadian timing of endogenous melatonin secretion or suppression of melatonin secretion by bright light has relevance for reproductive function in women is unclear.
Biological Rationale that Sleep May Modify Female Fertility
Apart from circadian timing, a bidirectional relationship may exist between sleep and female reproductive physiology. The findings detailed below provide evidence for a possible contribution of sleep, both quality and duration, to fertility.
Reproductive Hormonal Milieu Influences Sleep
Subjective sleep complaints are most common the days preceding and during menstruation [7, 10]; however, despite the subjective symptoms and dynamic changes in hormone levels, objectively measured sleep duration and quality remain relatively stable during the different phases of the ovulatory cycle [8, 10, 94, 96].
A few studies in normally cycling women have objectively demonstrated greater sleep fragmentation during the luteal phase compared to the follicular phase [11]. These findings have also been seen in women of later reproductive age [32, 131]. Interestingly, the predominant hormone during the luteal phase, progesterone, is a known GABA A receptor agonist [81] and exogenous administration increases sleep in post-menopausal women [25, 94] and women in the early follicular phase [106]. Conversely, endogenous progesterone is associated with increased wake time during the sleep period [10, 67, 68] potentially due to elevation of core body temperature [79]. In particular, the speed of progesterone rise and progesterone increases in the presence of estrogen may underlie the association of progesterone with reduced sleep quality [96].
Progesterone [96] may also be responsible for the increase in stage N2 sleep [34, 94, 96] seen during the luteal phase of the ovulatory cycle. Spindle frequency is also increased during the luteal phase but an association with progesterone has not been found [10]. Although stage REM sleep is reduced during the luteal phase [9, 10, 85, 94, 97], a direct association between REM sleep amounts and progesterone is less clear [10, 96].
In the follicular phase, at which time estradiol rises and peaks just prior to ovulation, core body temperature is lower and objective sleep quality may be superior to the luteal phase [11]. Estrogen improves sleep in post-menopausal women and one investigation demonstrated a positive association between actigraphically recorded sleep efficiency and an estrogen metabolite [67, 68]; conversely, a polysomnogram study demonstrated a positive association between wake after sleep onset and estrogen [10]. Therefore, the relationship between endogenous estrogen and sleep remains to be elucidated.
Sleep Influences Reproductive Hormones
Reciprocal to the impact of reproductive hormones on sleep, there is evidence that sleep modifies reproductive hormone levels.
Appropriate pulsatility of GnRH is a key factor in reproductive function and can be measured by LH pulsatility [119]. During puberty, sleep stimulates pulsatile gonadotropin secretion; however, in reproductive age women, sleep reduces LH pulse frequency during the early follicular phase and exerts minimal effect during other menstrual cycle phases [47]. Another key regulator of reproductive function is FSH, which stimulates growth of the ovarian follicle. A positive association between FSH and sleep duration was found in a study of 160 normally cycling, reproductive age women and persisted after adjusting for age and body mass index [118]. However, a causal relationship cannot be established and a study of sleep deprivation in the second half of the night did not result in FSH changes in women during the early follicular stage of the menstrual cycle [14]. The same investigation demonstrated that estrogen increased in response to partial sleep loss [14]. However, in a subsequent study, absolute sleep duration was not related to estradiol levels although greater variation in sleep duration was significantly correlated [73]. Another hormone active in reproductive physiology, prolactin, is well known to be augmented by sleep, largely independent of circadian timing [107, 114].
The above studies that evaluated the impact of sleep on hormone secretion have revealed contradictory findings and were often limited by small sample size. Further, the relevance of these changes in regards to fertility is unclear necessitating further investigation.
Other Potential Mechanisms that Implicate Sleep in Fertility
Psychological distress is considered detrimental to fertility; however, findings have been contradictory [71]. Further, study populations are often comprised of individuals seeking infertility treatment and investigations are typically cross sectional in design [71]. Therefore, directionality of the relationship cannot to be determined [71]. However, in a large prospective cohort study, women in the highest tertile of salivary alpha-amylase (a stress biomarker) exhibited a 29 % reduction in fecundity compared with women in the lowest tertile despite adjustment for age, race, income, and caffeine and tobacco use. Notably, frequency of intercourse and day of LH surge was similar in the high and low salivary alpha-amylase categories.
Psychological stress may negatively impact fertility through increased hypothalamic-pituitary-adrenal (HPA) axis activation and excessive sympathetic nervous system activity [24]. Sleep curtailment shares these biological outcomes of stress [2, 130]. Therefore, sleep loss could impact fertility through these mechanisms, or as sleep disruption often accompanies psychological stress, modify the relationship between psychological stress and infertility.
Additionally, another potential outcome of sleep deprivation, excessive oxidative stress is considered potentially detrimental to early reproductive outcomes including ovarian function, embryo implantation, and early pregnancy maintenance [93].
Finally, sleep may impact sexual behavior. In a recent investigation, sleep duration was associated with sexual desire and genital response [51]. With every 1-h increase in total sleep time, a 14 % increase in the odds of partnered sexual activity was seen [51]. Greater genital arousal was reported in women with longer average sleep time [51].
Evidence that Sleep and Circadian Disruption Impacts Fertility in Women
Despite the biological plausibility that sleep and circadian disruption could reduce fertility in women, data confirming this hypothesis are limited.
Shift Workers and Fertility
The vast majority of literature that associates sleep and circadian disruption with impaired reproductive health and fertility is among female shift workers. In shift work (particularly night work), the work period occurs when the circadian timing system promotes sleep and the time allotted for sleep overlaps with the time of high circadian alerting signal. Together, this results in sleep deprivation and misalignment between the endogenous circadian system and externally imposed light-dark cycle. This desynchrony has negative repercussions for physiological function as circadian oscillators are spread throughout the periphery [6].
A 2014 meta-analysis assessed the effect of shift work on the following reproductive outcomes: menstrual disruption defined as cycles less than 25 days or greater than 31 days, infertility defined as time to pregnancy greater than 12 months, and early spontaneous pregnancy loss prior to 24 weeks gestation [109]. Fifteen studies of more than 100,000 women in total were included [109]. Shift workers had increased odds of menstrual disruption (OR 1.22, 95 % confidence interval [CI] 1.15–1.29) and infertility (OR 1.8, 95 % CI 1.01–3.20) but not early spontaneous pregnancy loss. After confounder adjustment for age and various characteristics that differed among studies, including BMI, education, marital status, parity, race, smoking, caffeine, alcohol, physical activity, intercourse frequency, work hours, and other work related factors, the increased odds of menstrual disruption persisted, but the association with infertility was no longer significant. A subanalysis was performed and assessed night shift workers, defined as those working shifts only at night that typically began between 20:00 and 22:00 and lasted 10–12 h [109]. When restricting the analysis to night shift workers, a significantly increased risk of early spontaneous pregnancy loss was seen and persisted after adjustment for confounders (OR 1.41, 95 % CI 1.22–1.63).
A subsequent study demonstrated that nurses working night shifts more than 7 times per month were almost twice as likely to have short menstrual cycles (odds ratio of 1.76 95 % CI (1.32–2.28) [124]. Although menstrual cycle length is an important indicator of reproductive capacity, a recent investigation in 1739 women demonstrated no impact of shift work on fecundity; however, working more than 40 h per week was associated with a 20 % longer time to pregnancy [44]. Although monetarily compensated work time has the largest inverse relationship with sleep duration compared to other time use activities [13], self-reported sleep duration did not impact this finding [44]. Of note, frequency of intercourse was not measured [44]. A study of more than 2000 female flight attendants revealed a significantly increased risk of first trimester miscarriage when work times coincided with the sleep period based on time zone of origin [45].
Shift work has been used extensively as a surrogate marker for circadian desynchrony to assess health risks but this design is not without weaknesses. Vast differences in shift work schedules and duration of shift work may exist. Additionally, the degree of impairment during shift work likely reflects the degree of circadian desynchrony [46]. If individuals with impairment abandon shift work while individuals who tolerate non-traditional shifts remain in their current job, the healthy worker effect results [57, 58]. Therefore, we cannot assume that all individuals working shifts have the same amount of sleep deprivation and circadian misalignment. Further, it is unclear whether circadian desynchrony, sleep deprivation, or light at night mediate the observed risks. The contradictory results and overall minimal impact of shift work on early reproductive outcomes may be secondary to these weaknesses and this area deserves further attention.
Sleep Disturbances in Women Undergoing Infertility Treatment
Sleep disturbances are thought to be common in women undergoing infertility treatment [36] despite minimal data to support this hypothesis [69, 70, 84]. In one investigation evaluating a heterogeneous group of women in infertility clinic, 34 % answered yes to the question “do you experience disturbed sleep” [84]. Analysis with multivariable logistic regression (adjusting for race, BMI, and vasomotor symptoms) found that among premenopausal infertile women, those with diminished ovarian reserve were 20 times more likely to experience disturbed sleep. Sleep quality measured by the Pittsburgh Sleep Quality Index (PSQI) was also assessed in a group of women receiving infertility treatment with intrauterine insemination [69]. Poor quality sleep was found in 35 % [69]. When the same group evaluated the PSQI in 100 patients undergoing IVF, 23 % had poor quality sleep during oocyte retrieval and 46 % had poor quality sleep during embryo transfer [70]. Poor quality sleep was an independent predictor of psychological distress during the IVF procedure [70].
To our knowledge, we are the first group to evaluate sleep prior to and throughout the IVF process with both validated subjective and objective sleep measures (Goldstein et al. Sleep in women undergoing in vitro fertilization: a pilot study, submitted for publication). Actigraphically recorded sleep duration <7 h was noted in approximately 45–70 % women depending on which point in the cycle it was measured (Goldstein et al. Sleep in women undergoing in vitro fertilization: a pilot study, submitted for publication). Excessive daytime sleepiness measured by the Epworth sleepiness scale (ESS) was found in 20–30 % of women during the IVF process and poor sleep quality as defined by a PSQI value over 5 was seen in 30 % to nearly 60 % depending on time point. The most notable finding in our study was the trend for a linear association between baseline total sleep time (TST) and oocytes retrieved, an important outcome in IVF that is associated with live birth [112]. The number of oocytes retrieved increased by 1.5 on average for every 1-h increase in TST. TST, AMH, and day 3 FSH together explained 40 % of the variance of oocyte retrieved (Goldstein et al. Sleep in women undergoing in vitro fertilization: a pilot study, submitted for publication). This finding suggests, for the first time, that sleep duration may be a mediator of important markers of IVF success (Goldstein et al. Sleep in women undergoing in vitro fertilization: a pilot study, submitted for publication).
Polycystic Ovary Syndrome and Obstructive Sleep Apnea
Women with polycystic ovary syndrome (PCOS) have anovulatory infertility and early pregnancy loss. PCOS is associated with an increased risk of obstructive sleep apnea up to 30 times that of normal controls (122). The presence of obstructive sleep apnea in PCOS is a predictor of insulin resistance, glucose intolerance, and type 2 diabetes [115]. Additionally, treatment of obstructive sleep apnea improves insulin sensitivity in women with PCOS [116].
Insulin resistance in women with or without PCOS may contribute to infertility and recurrent pregnancy loss [29]. Therefore, evaluation and treatment of sleep-disordered breathing may have potential to improve fertility in this population of women. Further, as sleep deprivation also impairs glucose homeostasis [5], adequate sleep duration may also be crucial in this setting.
Conclusion
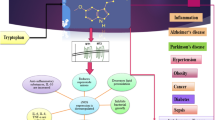
In summary, multiple pathways exist by which sleep and circadian rhythms may influence fertility (Fig. 1). Disrupted sleep may modify hormone secretion which could have negative repercussions on ovulatory cycles. Insufficient sleep duration or sleep disrupted by obstructive sleep apnea may result in insulin resistance and glucose intolerance potentially contributing to infertility and early pregnancy loss, particularly among women with PCOS. Further, disturbed or inadequate sleep may produce biological changes similar to psychological distress and could modify the relationship seen between stress and infertility. Although well described in animals, circadian control of the ovulatory cycle is less clear in humans. However, the effects of shift work and circadian clock gene polymorphisms on miscarriage and the finding of oscillation of core clock components in peripheral reproductive organs suggest a potential circadian contribution to conception.
Direct evidence and theoretical pathways of how disturbed sleep may result in infertility. Studies in female shift workers have demonstrated an increased risk of early pregnancy loss and abnormal menstrual cycle length, which may reflect problems with ovulation. As sex steroids and gonadotropins may demonstrate circadian rhythmicity and are modified by sleep, short sleep duration or circadian desynchrony could be detrimental to their normal secretion forming an intermediary pathway to poor early reproductive outcomes. Obstructive sleep apnea (OSA), short sleep duration, and circadian disruption are all known to result in glucose intolerance and insulin resistance, another potential link to infertility. The rhythmic oscillation of clock genes in the uterus may suggest circadian contributions to embryo implantation and early pregnancy. Increased hypothalamic-pituitary axis (HPA) activation, elevated sympathetic nervous system (SNS) activity, and excessive oxidative stress have been hypothesized as detrimental to multiple steps crucial to conception. These biological factors may all be influenced by sleep
Despite joint consensus recommendations for 7–8 h of sleep per night [125], Centers for Disease Control (CDC) survey data show that approximately 30 % of women 25–44 years of age report sleep less than or equal to 6 h in a 24 h day and the mean sleep duration in this age group is approximately 7.1 h [41]. Further, the prevalence of obesity has significantly risen in women over the past decade and is currently estimated at 40 % [40] which increases the risk of sleep-disordered breathing and associated metabolic abnormalities in women of child bearing age. Additionally, as we become an increasingly 24-h society, misalignment between our endogenous circadian rhythm and external light-dark schedule may impair reproductive health as evident in female shift workers. These epidemics underscore the need to understand the role of insufficient, disturbed, or mistimed sleep in infertility.
Finally, coincident with the increase in first births in women over 35 years of age, IVF use has more than doubled since 1996 [111]. Thirteen states have laws that require insurance companies to cover infertility treatment [108]. Despite the increased resources devoted to IVF, the procedure is still largely unsuccessful with >50 % of cycles failing to result in live birth [105]. Factors exacerbated by sleep deprivation [19, 38, 80, 130], including excessive sympathetic activation [1, 4, 104], oxidative stress [31, 49], and increased ghrelin [66] may be detrimental to IVF outcomes. Additionally, psychological distress, which could be mediated by poor sleep [70], may reduce IVF success [56] or result in discontinuation of the IVF process [43]. Therefore, sleep remains an under-investigated, modifiable target that may provide a non-pharmacological, cost-effective, and patient-centered avenue to improve IVF outcomes.
References
Ahn RS, Choi JH, Choi BC, Kim JH, Lee SH, Sung SS. Cortisol, estradiol-17β, and progesterone secretion within the first hour after awakening in women with regular menstrual cycles. J Endocrinol. 2011;211(3):285–95.
Akerstedt T, Perski A , Kecklund G. Sleep, occupational stress, and burn out. In Kryger, Roth, Dement (Eds.) Principles and practice of sleep medicine (p. 739). Philadelphia, PA: Elsevier; 2017.
Akiyama S, Ohta H, Watanabe S, Moriya T, Hariu A, Nakahata N, et al. The uterus sustains stable biological clock during pregnancy. Tohoku J Exp Med. 2010;221(4):287–98.
An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30(1):35–41.
Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, et al. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38(12):1849–60.
Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014;111(6):E682–91.
Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–43.
Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–22.
Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol. 1999;277(6 Pt 1):E1013–21.
Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–45.
Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30(10):1283–91.
Bao AM, Liu RY, van Someren EJ, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol. 2003;148(2):227–32.
Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–95.
Baumgartner A, Dietzel M, Saletu B, Wolf R, Campos-Barros A, Gräf KJ, et al. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res. 1993;48(2):153–78.
Beesley S, Lee J, Olcese J. Circadian clock regulation of melatonin MTNR1B receptor expression in human myometrial smooth muscle cells. Mol Hum Reprod. 2015;21(8):662–71.
Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139(6):1077–90.
Boden MJ, Varcoe TJ, Kennaway DJ. Circadian regulation of reproduction: from gamete to offspring. Prog Biophys Mol Biol. 2013;113(3):387–97.
Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204.
Boudjeltia KZ, Faraut B, Esposito MJ, et al. Temporal dissociation between myeloperoxidase (MPO)-modified LDL and MPO elevations during chronic sleep restriction and recovery in healthy young men. PLoS One. 2011;6(11):e28230.
Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977;198(1132):279–96.
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–17.
Bungum L, Jacobsson AK, Rosén F, Becker C, Yding Andersen C, Güner N, et al. Circadian variation in concentration of anti-Müllerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011;26(3):678–84.
Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril. 1998;70(1):56–9.
Campagne DM. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21(7):1651–8.
Caufriez A, Leproult R, L'Hermite-Balériaux M, Kerkhofs M, Copinschi G. Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women. J Clin Endocrinol Metab. 2011;96:E614–23.
Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544–77.
Christian CA, Mobley JL, Moenter SM. Proc Natl Acad Sci U S A. 2005;102(43):15682–7.
Chu A, Zhu L, Blum ID, Mai O, Leliavski A, Fahrenkrug J, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154(8):2924–35.
Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78(3):487.
Danilenko KV, Samoilova EA. Stimulatory effect of morning bright light on reproductive hormones and ovulation: results of a controlled crossover trial. PLoS Clin Trials. 2007;2(2):e7.
Das S, Chattopadhyay R, Ghosh S, et al. Reactive oxygen species level in follicular fluid—embryo quality marker in IVF? Hum Reprod. 2006;21(9):2403–7.
de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J Clin Endocrinol Metab. 2015;100(8):2918–26.
Dolatshad H, Campbell EA, O'Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod. 2006;21(1):68–79.
Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–35.
Endo A, Watanabe T. Effects of non-24-hour days on reproductive efficacy and embryonic development in mice. Gamete Res. 1989;22(4):435–41.
Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011;28(9):815–20.
Everett JW, Sawyer CH. A 24-hour periodicity in the "LH-release apparatus" of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218.
Faraut B, Bayon V, Leger D. Neuroendocrine, immune and oxidative stress in shift workers. Sleep Med Rev. 2013;17(6):433–44.
Fernando S, Osianlis T, Vollenhoven B, Wallace E, Rombauts L. A pilot double-blind randomised placebo-controlled dose-response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol. BMJ Open. 2014;4(8):e005986.
Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91.
Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 2015;38(5):829–32.
Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54(3):245–57.
Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? a systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18(6):652–69.
Gaskins AJ, Rich-Edwards JW, Lawson CC, Schernhammer ES, Missmer SA, Chavarro JE. Work schedule and physical factors in relation to fecundity in nurses. Occup Environ Med. 2015;72(11):777–83.
Grajewski B, Whelan EA, Lawson CC, Hein MJ, Waters MA, Anderson JL, et al. Miscarriage among flight attendants. Epidemiology. 2015;26(2):192–203.
Gumenyuk V, Howard R, Roth T, Korzyukov O, Drake CL. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep. 2014;37(3):545–56.
Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90(4):2050–5.
Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52(2):139–66.
Jana SK, Narendra Babu K, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29(4):447–51.
Johnson MH, Lim A, Fernando D, Day ML. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod Biomed Online. 2002;4(2):140–5.
Kalmbach DA, Arnedt JT, Pillai V, Ciesla JA. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12(5):1221–32.
Kennaway DJ, Varcoe TJ, Mau VJ. Rhythmic expression of clock and clock-controlled genes in the rat oviduct. Mol Hum Reprod. 2003;9(9):503–7.
Kerdelhué B, Brown S, Lenoir V, Queenan Jr JT, Jones GS, Scholler R, et al. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75(3):158–63.
Khattab AF, Mustafa FA, Taylor PJ. The use of urine LH detection kits to time intrauterine insemination with donor sperm. Hum Reprod. 2005;20(9):2542–5.
Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. Absence of circadian rhythms of gonadotropin secretion in women. J Clin Endocrinol Metab. 2011;96(5):1456–61.
Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675.
Knutsson A. Methodological aspects of shift-work research. Chronobiol Int. 2004;21(6):1037–47. 760.
Knuttson A, Akerstedt T. The healthy-worker effect: self-selection among Swedish shift workers. Work Stress. 1992;6(2):163–7.
Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006; 15 Spec No 2: 763R271-7.
Kovanen L, Saarikoski ST, Aromaa A, Lönnqvist J, Partonen T. ARNTL(BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 2010; 5(4).
Kripke DF, Elliott JA, Youngstedt SD, Parry BL, Hauger RL, Rex KM. Weak evidence of bright light effects on human LH and FSH. J Circadian Rhythms. 2010;8:5.
Kruijver FP, Swaab DF. Sex hormones are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75:296–305.
Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62.
Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–6.
Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15(1):71–83.
Li L, Ferin M, Sauer MV, Lobo RA. Serum and follicular fluid ghrelin levels negatively reflect human oocyte quality and in vitro embryo development. Fertil Steril. 2011;96(5):1116–20.
Li R, Cheng S, Wang Z. Circadian clock gene plays a key role on ovarian cycle and spontaneous abortion. Cell Physiol Biochem. 2015;37(3):911–20.
Li DX, Romans S, De Souza MJ, Murray B, Einstein G. Actigraphic and self-reported sleep quality in women: associations with ovarian hormones and mood. Sleep Med. 2015;16:1217–24.
Lin JL, Lin YH, Chueh KH. Somatic symptoms, psychological distress and sleep disturbance among infertile women with intrauterine insemination treatment. J Clin Nurs. 2014;23(11-12):1677–84.
Lin YH, Chueh KH, Lin JL. Somatic symptoms, sleep disturbance and psychological distress among women undergoing oocyte pick-up and in vitro fertilisation-embryo transfer. J Clin Nurs. 2016;25(11-12):1748–56.
Lynch CD, Sundaram R, Maisog JM, Sweeney AM, Buck Louis GM. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study--the LIFE study. Hum Reprod. 2014;29(5):1067–75.
McCormack CE. Acute effects of altered photoperiods on the onset of ovulation in gonadotropin-treated immature rats. Endocrinology. 1973;93(2):403–10.
Merklinger-Gruchala A, Ellison PT, Lipson SF, Thune I, Jasienska G. Low estradiol levels in women of reproductive age having low sleep variation.Eur J. Cancer Prev. 2008;17(5):467–72.
Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–73.
Moline ML, Albers HE. Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol Behav. 1988;43:435–40.
Moline ML, Albers HE, Todd RB, Moore-Ede MC. Light-dark entrainment of proestrous LH surges and circadian locomotor activity in female hamsters. Horm Behav. 1981;15(4):451–8.
Muter J, Lucas ES, Chan YW, Brighton PJ, Moore JD, Lacey L, et al. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J. 2015;29(4):1603–14.
Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab. 2008;295(5):E1025–31.
Nakayama T, Suzuki M, Ishizuka N. Action of progesterone on preoptic thermosensitive neurones. Nature. 1975;258:80.
Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obesity. 2014;21(4):293–8.
Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. nature reviews. Neuroscience. 2005;6(7):565–75.
Nishihara T, Hashimoto S, Ito K, Nakaoka Y, Matsumoto K, Hosoi Y, et al. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2014;30(5):359–62.
Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32(1):69–73.
Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15(6):1086–94.
Parry BL, Mostofi N, LeVeau B, Nahum HC, Golshan S, Laughlin GA, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. 1999;85(2):127–43.
Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135(4):559–68.
Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1-/- mice. Endocrinology. 2009;150(4):1879–85.
Reed BG, Carr BR. TheNormalmenstrual cycle and the control of ovulation. In: De Groot LJ, Beck-Peccoz P, Chrousos G, DunganK, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2015 May 22.
Reiter RJ, Tan DX, Tamura H, Cruz MH, Fuentes-Broto L. Clinical relevance of melatonin in ovarian and placental physiology: a review. Gynecol Endocrinol. 2014;30(2):83–9.
Reiter RJ, Tamura H, Tan DX, Xu XY. Melatonin and the circadian system: contributions to successful female reproduction. Fertil Steril. 2014;102(2):321–8.
Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. a prospective, clinical trial. Eur Rev Med Pharmacol Sci. 2010;14:55561.
Rosenwasser AM, Turek FW. Neurobiology of Circadian rhythm regulation. Sleep Med Clin. 2015;10(4):403–12.
Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14(4):345–57.
Schüssler P, Kluge M, Yassouridis A, Dresler M, Held K, Zihl J, et al. Progesterone reduces wakefulness in sleep EEG and has no effect on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2008;33(8):1124–31.
Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms. 2015;30(1):7–19.
Sharkey KM, Crawford SL, Kim S, Joffe H. Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med. 2014;15(6):688–93.
Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–56.
Shechter A, Lespérance P, Ng Ying Kin NM, Boivin DB. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. 2012;13(8):1071–8.
Shibui K, Uchiyama M, Okawa M, Kudo Y, Kim K, Liu X, et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48(11):1062–8.
Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012;97(5):1039–43.
Simonneaux V, Bahougne T. A multi-oscillatory Circadian system times female reproduction. Front Endocrinol (Lausanne). 2015;6:157.
Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500.
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–66.
Smeenk JM, Verhaak CM, Vingerhoets AJ, Sweep CG, Merkus JM, Willemsen SJ, et al. Stress and outcome success in IVF: the role of self-reports and endocrine variables. Hum Reprod. 2005;20(4):991–6.
Society for assisted reproductive technology. National Summary Report. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0. Accessed 27 June 2016.
Söderpalm AH, Lindsey S, Purdy RH, Hauger R, de Wit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–54.
Spiegel K, Follenius M, Simon C, et al. Prolactin secretion and sleep. Sleep. 1994;17:20–7.
State Laws Related To Insurance Coverage For Infertility Treatment. http://www.ncsl.org/research/health/insurance-coverage-for-infertility-laws.aspx. Accessed 12 Jan 2016.
Stocker LJ, Macklon NS, Cheong YC, Bewley SJ. Influence of shift work on early reproductive outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(1):99–110.
Summa KC, Vitaterna MH, Turek FW. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS One. 2012;7(5):e37668.
Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance - United States, 2013. MMWR Surveill Summ. 2015;64(11):1–25.
Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7.
Van Cauter and Tasali. Endocrine physiology in relation to sleep and sleep disturbances. In: Kryger, Roth, Dement (Eds.) Principles and practice of sleep medicine (p. 205). Philadelphia, PA : Elsevier. 2017.
Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(10):3878.
Tasali et al. Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. Clin Endocrinol Metab. 2011; 96(2): 365.
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–31.
Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77(4):738–44.
Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729–37.
Uchikawa M, Kawamura M, Yamauchi N, Hattori MA. Down-regulation of circadian clock gene period 2 in uterine endometrial stromal cells of pregnant rats during decidualization. Chronobiol Int. 2011;28(1):1–9.
Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011;27(11):857–61.
Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86(2):517–20.
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719–25.
Wang Y, Gu F, Deng M, Guo L, Lu C, Zhou C. Rotating shift work and menstrual characteristics in a cohort of Chinese nurses. BMC Womens Health. 2016;16(1):24.
Watson NF, Badr MS, Belenky G. Joint consensus statementof the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–83.
Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31(2):147–57.
WHO Technical Report Series. Recent advances in medically assisted conception number 820, 1992, 1–111.
Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–21.
Williams 3rd WP, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol. 2012;3:60.
Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, et al. Relationship of sleep quantity and quality with 24-hour urinarycatecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34(2):225–33.
Zheng H, Harlow SD, Kravitz HM, Bromberger J, Buysse DJ, Matthews KA, et al. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: Study of Women's Health Across the Nation Sleep Study. Menopause. 2015;22(1):66–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Cathy A. Goldstein and Yolanda R. Smith declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Women and Sleep
Rights and permissions
About this article
Cite this article
Goldstein, C.A., Smith, Y.R. Sleep, Circadian Rhythms, and Fertility. Curr Sleep Medicine Rep 2, 206–217 (2016). https://doi.org/10.1007/s40675-016-0057-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-016-0057-9