Abstract
Haloxylon ammodendron is an excellent windproof and sand-fixing species whose shrubs are widely cultivated in arid desert areas of northwest China but are now at risk of degradation and shrinkage. Using the chlorophyll fluorescence image analysis technique, the response of photosystem II (PSII) photochemical efficiency and non-photochemical quenching capacity to sediment-carrying wind and sand-free wind (both 12 m s−1) lasting for 10, 20, and 40 min were studied with seedlings in a wind tunnel. The results indicated that the sand-free wind had little influence on the maximum quantum efficiency of PSII photochemistry, Fv/Fm, which was approximately 0.80 on average; however, the Fv/Fm decreased over exposure time in the sediment-carrying wind group, with values smaller than those in the wind-only group. The non-photochemical quenching was sensitive to wind erosion, and sediment-carrying wind could aggravate the reduction in non-photochemical quenching (NPQ). Except for the 10 min duration, the maximum quantum efficiency of PSII photochemistry after dark recovery for 15 min, Fvr/Fmr, was lower in seedlings exposed to sediment-carrying wind than in those in the wind-only group. Compared to wind-only, wind-blown sand led to a water imbalance and withering in seedlings, causing the concentration of photosynthetic pigments (when based on the fresh mass of green branches) to not decrease. With a longer exposure time to sediment-carrying wind, both the probability and extent of lignified spots occurring increased in green assimilative branches. Our results demonstrated that sediment-carrying wind at 12 m s−1 lasting for 20 min or more could cause irreversible damage to the photosynthetic apparatus of H. ammodendron seedlings. Therefore, frequent and strong sandstorms are the main disturbance factors leading to shrinkage of shrubs and limiting their self-renewal.
Similar content being viewed by others
1 Introduction
A sandstorm is a weather phenomenon linked to desertification that is caused by wind erosion, and its formation and intensity is influenced by both natural factors and human activities (Wang et al. 2006). As a very common type of sudden weather disturbance in arid and semiarid desert areas of northwest China, sandstorm outbreaks are frequent in winter and early spring (Zhao and Sun 2013). Spring is a sensitive time of the year for seed germination and seedling settlement and is also a key period for vegetation population renewal. Frequent and strong outbreaks of sandstorms are very harmful and destructive to fragile desert ecosystem (Shi et al. 2000); such disturbances can severely restrict the sustainable development of arid and semiarid desert areas and adversely affect the restoration and reconstruction of their ecological environment (Dong et al. 2004; Zhang et al. 2010; Qu et al. 2019).
Wind-blown sand or sediment-carrying winds lead to the migration of loose sand particles near the surface (Li and Ni 1998; Ogunjobiet et al. 2003). Their impact on desert plants can manifest as mechanical injury caused by wind erosion and the hitting of sand grains, which damages the tender epidermis tissue and leads to the outflow of cell fluid (Anten et al. 2010); this injury is especially severe at the vulnerable stage of young plantlets, often resulting in seedling death (Zhao et al. 2017). It is generally believed that the process by which sediment-carrying wind injures plants includes variation, from quantitative to qualitative, in their physiological functioning; put differently, their physiological functions respond to this abiotic disturbance only appears after a certain duration (Retuerto and Woodward 1993; Henry and Thomas 2002). Research on the eremophyte Sarcozygium xanthoxylon Bunge indicated that mild and moderate sediment-carrying wind could cause damage to the seedlings, but not necessarily osmotic stress; however, severe wind-blown sand was able to increase the activity of superoxide dismutase and catalase in leaves and to increase their contents of soluble sugar and proline, which led to cell fluid outflow under osmotic stress (Li et al. 2019). Nevertheless, it was unclear whether this constituted irreversible damage to seedlings or whether defining a key physiological damage threshold is needed.
Photosynthesis is a fundamental physiological process of green plants, with many mechanisms that ensure the efficient operation of the photosynthetic apparatus in the face of adversity. Early research indicated that strong wind can decrease the photosynthetic rate and increase the transpiration rate of plants (Qu et al. 2009); compared with sand-free wind, their net photosynthetic rate was significantly affected by wind-blown sand, especially in herbs, whereas shrubs have a better resistance to strong winds (Yu et al. 2002). Enhancing the level of non-radiative heat energy dissipation could occur as a result either of processes that protect the leaf from light-induced damage or of the damage itself (Maxwell and Johnson 2000). This response is considered an important regulatory approach by which plants tolerate environmental adversity (Niyogi and Truong 2013). Reduced photosynthesis inevitably leads to less demand for available light energy, resulting in a surplus of light energy in the photosynthetic apparatus; hence, adversity in the form of a sandstorm might also induce an accumulation of excess excitation energy.
Haloxylon ammodendron (C. A. Mey.) Bunge is a super xerophytic shrub or small tree of the Chenopodiaeceae family, belonging to the Asian desert component, widely distributed in the desert region of Central Asia and sporadically distributed in northwest China. Due to its advantages of drought resistance, barren resistance, sand buried wind erosion resistance, salt and alkali resistance, and characteristics of easy survival and fast growth, it has been widely used in desertification control in arid desert areas of northwest China. It is considered an important plant resource for windproofing, water and soil conservation, and saline-alkali soil improvement, with high economic and ecological value (Wang and Ma 2003). Many studies of this eremophyte are available, mainly on its seed germination (Tobe et al. 2000; Wang and Zhao 2015), phenology (Jiang et al. 2017), physiological and ecological adaptability (Ju et al. 2005; Tian et al. 2011), and applied use in ecological protection and restoration (Bedunah and Schmidt 2006) and expression of drought-resistant genes (Han et al. 2016). Surprisingly, however, only a few studies have investigated the adaptive traits and response of H. ammodendron to sandstorms.
As a typical desert shrub, H. ammodendron is an excellent windproof and sand-fixing plant widely cultivated in mobile dunes, semifixed dunes and fixed sand dunes of the oasis edge of Minqin in Gansu Province (China). However, due to the adverse natural habitat and other reasons, the early-built artificial shrubs began to shrink or even die, and some of the fixed dunes began to activate, which brought new hazards to the lives of local residents and economic production (Zhang et al. 2009). It is widely thought that a lower underground water level, soil water scarcity, and higher soil salt content in these afforestation areas are the main factors driving the degradation of H. ammodendron, which leads to their limited ability to utilize water and poor population regeneration (Si et al. 2011). According to an earlier field investigation, it was difficult to observe surviving young seedlings in adult stands; the age structure of the population stably decayed during the shrinkage of the H. ammodendron plantation (He et al. 2017). An insufficient number of progeny and recruitment from seedlings into adults to maintain minimum population density may be a major reason limiting the self-renewal of plant populations and can even cause their degeneration (Bormann and Likens 1981; Edelfeldt et al. 2019). Therefore, we proposed the following hypothesis: green assimilative branches of H. ammodendron seedlings are sensitive to frequent and strong sandstorms in early spring, thereby incurring irreversible damage to their photosynthetic apparatus that disrupts their physiological functioning. To test this hypothesis, we set up wind tunnel simulation experiments to study the effects of wind-blown sand on photosynthesis by measuring the changes in photochemical efficiency of the PSII reaction center of H. ammodendron seedlings using a wind-only group as a reference (i.e., control). This approach, combined with field observations of plant morphological characteristics, allows us to expound upon the potential mechanism by which the photosynthetic apparatus is damaged by sandstorm disturbances.
2 Material and methods
2.1 Plant material and sand-blowing wind tunnel simulation test
Seeds were collected in November 2016 from a field of H. ammodendron plantation in Minqin County, Gansu Province, China. In early May 2017, seedlings were raised in the experimental nursery of the Gansu Desert Control Research Institute in Wuwei City. Plastic pots with a 15 L volume (an opening diameter of 22 cm and a height of 45 cm) were used; into these pots, the cultivation substrate was added in a 1:9 ratio of loam to sand, with a small amount of organic fertilizer applied, and approximately 10 seeds were planted per pot. One plot (approximately 5 m × 8 m) with good ventilation and lighting was selected, and all pots were arranged in 5 rows in the east–west direction. The spacing between pots was approximately 8 cm in each row, the row spacing was approximately 50 cm, and each pot was buried in soil to a depth of approximately 40 cm to avoid high temperature. Normal field management was conducted during the plant growing period, and seedlings were thinned to 4 to 5 plants per pot after they had germinated to approximately 5 cm height. Wind blowing simulation experiments were performed from July to September 2017; from August to September 2018, the same experiments were performed again with spare biennial seedling plants.
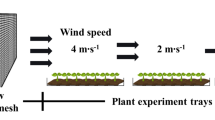
The wind erosion simulation experiment was carried out in the wind tunnel for blown sand of the State Key Laboratory Breeding Base of Desertification and Aeolian Sand Disaster Combating. The sand used in this experiment was taken from the Badain Jaran Desert marginal dune, located near the Gansu Minqin National Studies Station for Desert Steppe Ecosystem. The raw sands were filtered through wire mesh, and then fine sands were blown in the wind tunnel to remove dust. The total length of the tunnel is nearly 39 m; its test section is 16 m long, with a cross-section of 1.2 m × 1.2 m; the axis wind speed can be controlled from 4 to 35 m s−1 and applied continuously, with accuracy between 0.5% and 3.0%. The simulation experiment was divided into two processing treatments: wind-only (sand-free wind) and sediment-carrying wind. The fine sands were laid at the test section, approximately 10 m long and 10 cm thick (Sun et al. 2021). Plastic pots of plants treated with sand-free wind were placed in front of the sand-bed section, approximately 1 m from the front edge of the sand bed; pots exposed to wind-blown sand were placed in part of the tunnel’s moving section after the sand bed. In the moving section, the pot could be adjusted up and down to ensure that the new branches were completely within 30 cm of the surface of the sand bed. The wind speed was set at 12 m s−1, and three blowing durations of 10, 20, and 40 min were applied for both treatments.
2.2 Chlorophyll fluorescence images
After completing the simulation trial in the wind tunnel, the experimental potted plants were moved onto the ground under semishaded trees mainly with scattered light, and the photosynthetic photon flux density (PPFD) was 400 ± 100 μmol photons m−2 s−1 to avoid direct sunlight. To remove any fine sand particles clinging to the surface of these plants’ branches, a small hand-held hair dryer was used to quickly blow air over the whole plant. Then, branches growing at a similar height and position were collected randomly from different treated plants, ensuring that they were free of any damage (visually assessed). Next, a chlorophyll fluorescence image analyzer (CF imager, Technologica Ltd, United Kingdom) was used to determine the fluorescence parameters of green assimilating shoots of H. ammodendron seedlings. From each treatment group, more than 10 green branch segments, each nearly 8 cm in length, were placed on the imaging platform of the chlorophyll fluorescence image analyzer with a fully wet cotton yarn pad laid beneath them. The green branches were immobilized using a 0.09-mm diameter nylon line and brought into focus. Given the experimental aim, a preset protocol was run to determine the chlorophyll fluorescence images under a differing steady-state PPFD. During the measurement period, the indoor air temperature was maintained at 18.0 ± 1.0 °C, and the relative air humidity was 40 ± 2%.
2.2.1 The protocol
2.2.1.1 Steady-state PSII photochemical efficiency
The built-in protocol was divided into two parts to determine chlorophyll fluorescence parameters by using steady-state actinic light. After 15 min of dark adaptation, the minimum chlorophyll fluorescence yield (Fo) in the opened PSII reaction center and the maximum chlorophyll fluorescence yield (Fm) in the closed state were determined; to do this, low steady-state actinic light (400 μmol photons m−2 s−1, LL) and high steady-state actinic light (1500 μmol photons m−2 s−1, HL) were alternately turned on, for which light processing was performed for total 30 min. The maximum fluorescence yield (Fm′) and the steady-state fluorescence yield (Fs) were determined every 5 min under continuous illumination for each PPFD. After irradiating the plant samples for a total of 30 min (at low and high PPFDs), they underwent a dark recovery for 15 min, during which time the minimum and maximum chlorophyll fluorescence yield, respectively For and Fmr, were measured successively at 5, 10, and 15 min. The saturation pulse light used for the determination of Fm, Fm′ and Fmr was 6840 µmol photons m−2 s−1 and lasted for 60 ms.
2.2.1.2 Light response curve
Following Baker and Rosenqvist (2004), both Fo and Fm were determined after 15 min of dark adaptation; then, after irradiation with low steady-state actinic light (400 μmol photons m−2 s−1) for 10 min, the different PPFDs were varied successively to determine Fs and Fm′. Overall, 12 PPFD levels were set and applied: 25, 50, 100, 150, 200, 300, 500, 800, 1000, 1200, 1500, and 1800 μmol photons m−2 s−1; the saturation pulse light of Fm and Fm′ was measured for each at 6840 μmol photons m−2 s−1 (lasting for 60 ms). Depending on how the trend of Fs changed, the equilibrium time at a low PPFD of 25, 50, 100, and 150 μmol photons m−2 s−1 was determined to be 2 min; likewise, the equilibrium time for each PPFD level greater than 200 μmol photons m−2 s−1 was set to 1 min.
2.2.2 Assimilation branch localization and image analysis
In the graphic editing phase of fluorescent imagery, the images of green assimilative branches of H. ammodendron seedlings treated with two wind erosions (i.e., without vs. with sand) and three exposure durations (i.e., 10, 20, or 40 min) were located and separated, one by one, and these fluorescence images were resolved into the data values of chlorophyll fluorescence parameters. More details on the specific operational steps can be found in the CF imager manual (http://www.technologica.co.uk/).
2.2.3 Analysis of PSII photochemical efficiency and quenching of excess excitation energy
The maximum quantum efficiency of PSII photochemistry (Fv/Fm) was calculated using Fo and Fm obtained after 15 min of dark adaptation, where Fv = Fm–Fo. The maximum quantum efficiency of PSII photochemistry (Fvr/Fmr) after dark relaxation recovery lasting 5, 10, and 15 min was calculated using the chlorophyll fluorescence yield values, For and Fmr, at each corresponding time, where Fvr = Fmr – For.
The PSII maximum efficiency (Fv′/Fm′) and the PSII operating efficiency (Fq′/Fm′) under steady-state actinic light were calculated using the following formulas (Genty et al. 1989): Fv′/Fm′ = (Fm′−Fo′)/Fm′; Fq′/Fm′ = (Fm′−Fs)/Fm′. The PSII efficiency factor (Fq′/Fv′) and the non-photochemical quenching (NPQ) were calculated according to the formulas of Bilger and Björkman (1990): Fq′/Fv′ = (Fm′−Fs)/(Fm′−Fo′) and NPQ = Fm/Fm′−1. The Fo′ in the above formulas was estimated by applying the empirical formula of Oxborough and Baker (1997): Fo′ = Fo/(Fv/Fm−Fo/Fm′), wherein Fo and Fm are the chlorophyll fluorescence yield values of dark adaptation for 15 min.
2.3 Measurement of photosynthetic pigments
The concentration of photosynthetic pigments was determined by spectrophotometry. After applying the wind-only and sediment-carrying wind treatments to the plants, their green assimilative branches from different exposure durations were immediately sampled. The upper part of the branchlets was taken and cut into approximately 1-cm-long segments, after which an accurately weighed amount of 250 mg was promptly immersed in a brown glass bottle containing 20 mL of acetone and ethanol extract solution (acetone: ethanol: water = 4.5:4.5:1). After closing its cap, each bottle was allowed to soak in a low-temperature room to avoid light irradiation until the green branch segments became colorless (Shi et al. 2004). Six repetitions were used for each treatment; please refer to Wellburn (1994) for details on the determination and calculation of chlorophyll concentration.
2.4 Measurement of water content
After imposing the wind treatments, the green assimilative branches were immediately collected from plants among the different exposure durations. The upper part of the branchlets was taken and cut into approximately 1-cm-long segments; 500 mg of this segment was accurately weighed and immediately placed in an electric oven to dry for 1 h at 100 °C and then at 80 °C for 24 h. Dry mass was recorded; the water content of green branches was calculated as follows: water content (%) = (fresh mass−dry mass)/fresh mass. Five replicates were set for each of the 10-, 20-, and 40-min durations per treatment.
2.5 Morphological observations
After simulation of wind erosion in the wind tunnel, the pots were moved to the original cultivation area and still buried in the soil approximately 40 cm deep again. Plant morphological changes were observed continuously on the first, second, and third days of wind erosion, and injuries to plants and branches were noted and documented visually using a digital camera; pictures were image cropped to eliminate background clutter (Fig. 8).
2.6 Data analysis
After preprocessing the data using Microsoft Excel software, they were statistically analyzed in SPSS 16.0 software. An independent sample t test was used to determine the difference in the response variable between the wind-only and sediment-carrying wind treatments at a given exposure duration. To analyze the differences among the three wind exposure durations, as well as the subsequent three relaxation times, multivariate ANOVA was used; the least significant difference (LSD) test was used for the multiple comparisons of means, with a significance level set to α = 0.05. Coefficients of variation (CV) were estimated as the ratio of samples’ standard deviation to its mean value. Microsoft Excel software was used to collate data and draw the graph, for which the data shown are the mean value, and the error bar is the standard deviation (SD) except the standard error (SE) in Fig. 1.
3 Results
3.1 Rapid light curve of chlorophyll fluorescence parameters
The variation of light response curves indicated that whether the PSII operating efficiency (Fq′/Fm′) or efficiency factor (Fq′/Fv′) differed little between the sand-free wind and sediment-carrying wind, and no apparent changes with prolongation of wind blowing were observed. The PSII maximum efficiency (Fv′/Fm′) in the sediment-carrying wind group was slightly lower than that in the wind-only group; the light response curves of Fv′/Fm′ tended to stabilize in both wind treatment groups when the photosynthetic photon flux density (PPFD) was higher than 300 μmol photons m−2 s−1 (Fig. 2).
Light response curves of the PSII operating efficiency (Fq′/Fm′), PSII efficiency factor (Fq′/Fv′), and PSII maximum efficiency (Fv′/Fm′) in green assimilative branches of H. ammodendron seedling after their exposure to wind-only and sediment-carrying wind blowing at 12 m s−1 for 10, 20, and 40 min. Each point of data is the mean of 10 samples; the standard deviation was omitted in the figure because it would obscure the means and trends shown
The non-photochemical quenching (NPQ) decreased with increasing wind erosion time (Fig. 1). The NPQ, however, was obviously lower in the sediment-carrying wind group than in the wind-only group for a given exposure duration; even with just 10 min of exposure to blown sediment-carrying wind, the curve of NPQ remained lower than that obtained after 40 min of exposure to wind-only conditions.
3.2 Photochemical efficiency and non-photochemical quenching of PSII reaction center
The measurements under steady-state actinic light demonstrated a consistent effect on all chlorophyll fluorescence parameters between low and high light PPFD (400 and 1500 μmol photons m−2 s−1; expressed as LL and HL, respectively). Compared with the wind-only group, the blowing of sediment-carrying wind caused a reduction in Fv′/Fm′; moreover, after continuous blowing for 20 min and 40 min, the corresponding Fv′/Fm′ values in the sediment-carrying wind group were lower (p < 0.01 and p < 0.05, respectively) than those in the wind-only group. With prolongation of wind erosion, the Fv′/Fm′ slightly decreased in the sediment-carrying wind group and slightly increased in the wind-only group; there was a difference in Fv′/Fm′ between 40 and 10 min of exposure to blowing when measured at LL intensity (p < 0.05). Compared with sand-free wind, the Fq′/Fm′ decreased in seedlings exposed to sediment-carrying wind, while Fq′/Fv′ increased slightly; both Fq′/Fm′ and Fq′/Fv′ was no consistent or exhibited clear changes with the duration of wind erosion (Fig. 3).
Effects of wind-only and sediment-carrying wind blown at 12 m s−1 on the PSII operating efficiency (Fq′/Fm′), PSII efficiency factor (Fq′/Fv′), and PSII maximum efficiency (Fv′/Fm′) of H. ammodendron seedlings after irradiation at low and high PPFDs for 15 min. Different capital and lowercase letters indicate significant difference in Fq′/Fm′, Fv′/Fm′ or Fq′/Fv′ among 10-, 20-, and 40-min exposures within the wind-only and sediment-carrying wind treatments, respectively (α = 0.05); “ns” indicates no significant difference (p > 0.05), “*” indicates significant difference (p < 0.05), and “**” and “***” indicate highly significant difference (p < 0.01 and p < 0.001, respectively) between the wind-only and sediment-carrying wind treatments
The values of NPQ were markedly reduced (10 min, p < 0.05; 20 min, p < 0.01; 40 min, p < 0.001) after sand blowing compared with the wind-only group (Fig. 4). NPQ declined in both wind treatments with the prolongation of wind erosion; differences were evident between 10 and 40 min of exposure to sediment-carrying wind blowing under either an LL or HL intensity (p < 0.05).
Effects of wind-only and sediment-carrying wind blown at 12 m s−1 upon non-photochemical quenching (NPQ) of H. ammodendron seedlings after irradiation at low and high PPFDs for 15 min and their variation within exposure durations of 10, 20, and 40 min to blowing wind. Different capital and lowercase letters indicate significant differences in NPQ among 10, 20, and 40 min of exposure within the wind-only and sediment-carrying wind treatments, respectively (α = 0.05); “*” indicates significant difference (p < 0.05), and “**” and “***” indicate highly significant difference (p < 0.01 and p < 0.001, respectively) between the wind-only and sediment-carrying wind treatments
3.3 Maximum quantum efficiency of PSII
After dark adaptation for 15 min, the maximum quantum efficiency of PSII photochemistry (Fv/Fm) was similar among the 10, 20 and 40 min blowing of sand-free wind, for which the average values were approximately 0.80. Nevertheless, with prolongation of wind erosion, Fv/Fm gradually decreased in the sediment-carrying wind group, with difference between durations of 10 min and 40 min (p < 0.05). Compared with the wind-only group, sediment-carrying wind induced a remarkable reduction (10 min, p < 0.05; 20 and 40 min, p < 0.01) in Fv/Fm (Fig. 5).
Response of the maximum quantum efficiency of PSII photochemistry (Fv/Fm) in H. ammodendron seedlings to wind-only and sediment-carrying wind blowing at 12 m s−1 and their variation within exposure durations of 10, 20, and 40 min to blowing wind. Different capital and lowercase letters indicate differences in Fv/Fm among 10, 20, and 40 min of exposure within the wind-only and sediment-carrying wind treatments, respectively (α = 0.05); “*” and “**”, respectively, indicate significant difference (p < 0.05) and highly significant difference (p < 0.01) between wind-only and sediment-carrying wind treatments
After irradiation with steady-state LL and HL intensity for a total of 30 min, dark relaxation measurements (dark recovery) were taken. In Fig. 6, the prolonged dark recovery provoked the maximum quantum efficiency of PSII photochemistry after dark relaxation (Fvr/Fmr) to be gradually recovered in the seedlings, regardless of whether they were treated with sand-free wind or sediment-carrying wind. When previously windblown for 10 min and 40 min, we observed prominent differences in Fvr/Fmr between the 5 min and 15 min of dark relaxation in the wind-only group (p < 0.05). After experiencing 10 min of wind erosion, the Fvr/Fmr of seedlings in the sediment-carrying wind group seemed slightly higher than those in the wind-only group, albeit not a significant difference. In contrast, after 20 min and 40 min, the corresponding values of Fvr/Fmr were markedly lower in the sediment-carrying wind group (p < 0.05; p < 0.01; p < 0.001).
Comparison of the maximum quantum efficiency of PSII photochemistry after dark relaxation (Fvr/Fmr) in H. ammodendron seedlings between wind-only and sediment-carrying wind treatments blown at 12 m s−1 for 10, 20, and 40 min and their variation at 5, 10, and 15 min of dark relaxation. Different capital and lowercase letters indicate significant differences in Fvr/Fmr among 5, 10, and 15 min of dark relaxation time for the wind-only and sediment-carrying wind treatments, respectively (α = 0.05); “ns” indicates no significant difference (p > 0.05), “*” indicates significant difference (p < 0.05), and “**” and “***” indicate highly significant differences (p < 0.01 and p < 0.001, respectively) between the wind-only and sediment-carrying wind treatments
3.4 Photosynthetic pigments and water content
Apart from the chlorophyll b (Chl b), the concentration of total chlorophyll (Chl), carotenoid (Car), and chlorophyll a (Chl a) increased with exposure duration to sediment-carrying wind, and all were higher in seedlings sand-blown for 40 min (p < 0.05) (Table 1). The ratio of carotene to total chlorophyll (Car/Chl) was higher after incurring wind blowing for 20 than 40 min (p < 0.05). The ratio of chlorophyll a to b (Chl a/b) was also slightly higher in seedlings subjected to wind-blown sand for 20 min, although no differences among the three exposure durations were found.
When compared with 10 min of exposure, the blowing of sediment-carrying wind caused significant loss in the water content of green branches at 20 and 40 min (p < 0.05), but in the wind-only group, the water content was greater after 20 and 40 min of exposure than just 10 min of exposure (p < 0.05) (Fig. 7). Compared with the wind-only group, the water content was reduced in a remarkable way after incurring blowing sand for 20 and 40 min (p < 0.001).
Effects of wind-only and sediment-carrying wind blown at 12 m s−1 on the water content of H. ammodendron seedlings and their variation within exposure durations of 10, 20, and 40 min to blowing wind. Different capital and lowercase letters indicate significant differences in the water content among 10, 20, and 40 min of exposure within the wind-only and sediment-carrying wind treatments, respectively (α = 0.05); “ns” indicates no significant difference (p > 0.05), and “***” indicates extremely significant difference (p < 0.001) between the wind-only and sediment-carrying wind treatments
3.5 The surface structure of green assimilative branches of seedlings
The morphological characteristics indicated no variation in green assimilative branches of seedlings when sand-free wind blew for 10 min; however, when exposed to sediment-carrying wind, dead color and/or lignified spots occasionally appeared in some tender branches but seemingly did not affect adversely their survival. In some cases, casually wilting or drooping branches after 20 min of sand-free wind blowing were observed, but these withering branches could restore themselves to normal growing status after one night of recovery; it was more common that some green branches become darkened when the plants were exposed to the sediment-carrying wind for 20 min, their branches forming lignified spots and a few days later some of the branches were occasionally dry and dead. Experiencing 40 min of wind-only blowing could cause a sporadic wilting phenomenon in some green branches, yet most of them reverted to normal growth the next day; however, when exposed to sediment-carrying wind, the tender branches often withered within a few hours, and most of them or even the whole plant died a day later.
The blowing of sediment-carrying wind often causes mechanical injury to the branch surface of H. ammodendron seedlings. With prolonged wind erosion, the probability and degree of incurring such injured branches increased. As evidenced by Fig. 8, lignified spots caused by sediment-carrying wind mainly appeared at the branch base of relatively mature or young twigs, with more serious injuries appearing on the windward side of their branches.
Morphological changes to the green branches of H. ammodendron seedlings after they were exposed to sediment-carrying wind blown at 12 m s−1 for 10, 20, and 40 min and measured 2 days later. Lower-case letters a, b, and c are the photos taken 10 min after the wind-blowing treatment; likewise, d, e, and f were taken after 20 min; and g, h, and i after 40 min
4 Discussion
4.1 Sediment-carrying wind worsened the water imbalance in H. ammodendron seedlings
A wind speed of 12 m s−1 an be considered strong wind for plants; it easily blows sand from the dry and loose soil surface in the absence of any vegetation cover, forming wind-blown sand or sandstorm weather (Zhan et al. 2009). In field observations during a sandstorm, sand particles were mainly concentrated at heights of less than 30 cm near the ground (especially below 10 cm), and the sand transport rate between 0 and 20 cm was approximately 45 g cm−2 (Sun et al. 2021); sand particles are apparently more hazardous to newly germinated seedlings. The study simulated in a wind tunnel found that, compared with the wind-only group, the sediment-carrying wind was more likely to cause irreversible damage to H. ammodendron seedlings. Even when blown by sand-free wind, the green assimilative branches occasionally underwent the phenomenon of water loss and wilting, but these deficiencies could be restored to the normal growing status after one night of recovery. The sediment-carrying wind provoked a conspicuous impact, forming lignification spots on the tender green assimilative branches, most of which emerged a day later; if it lasted for 40 min, clusters of lateral branches darkened and wilted, and most of them soon became dry and fell off easily, or even whole plant died.
Zhao et al. (2015) argued that strong wind could lead to water stress in Pinus sylvestris var. mongolica seedlings, damaging their cell membrane structure and function, and they considered that it is the main reason why the growth of plants is restricted in desert areas. Our observations indicated that the green assimilative branches of H. ammodendron seedlings were often characterized by the phenomenon of wilting and/or withering, and this was particularly prominent in those exposed to sediment-carrying wind. Direct measurements also showed that the water content of green branches could remain relatively stable when blown upon by sand-free wind; its average was 74.21% with a coefficient of variation (CV) of 3.5% (Fig. 7), but the blowing of wind-blown sand would lead to their water imbalance and the wilting phenomenon. The occasional wilting and sagging phenomenon of the green assimilative branches that appeared in the wind-only group was probably related to their growth position or perhaps due more to the thinning of the diffusion layer on the branch surface. The sediment-carrying wind led to an imbalance in water status, in that seedlings’ water contents were lower after blowing for 20 min and 40 min; the probability and degree of withering was exacerbated by longer blowing duration, and the average water content was 67.11% with a CV of 6.82%. Obviously, this resulted from the impact of sand particles, probably from damage to epidermal tissue, causing the outflow of cell fluid in the tender branches. A slightly increased water content was also observed after the 20-min exposure to sediment-carry wind blowing in similar experiments. However, the wind-blown sand that lasted for 40 min could definitely lead to a lower water content, thus rendering plants more prone to wilting.
Given that sediment-carrying wind could cause a continuous decline in the water content of H. ammodendron seedling tender branches, their photosynthetic pigments might not be oxidized and degraded so quickly. A decrease in the concentration of photosynthetic pigments, based on fresh mass unit, was not observed after erosion of wind-blown sand; in contrast, its concentration could increase after the blowing of sediment-carrying wind at 40 min (Table 1). These results demonstrated that sustained sediment-carrying winds do not diminish the concentration of photosynthetic pigments, mainly due to the lowered water content of green assimilative branches.
Usually, light wind or breezing causes the diffusion layer to thin and disappear, decreasing the external diffusion resistance and thereby promoting transpiration (Yu et al. 2002). The effect of a strong wind is greater than that of a breeze, which may lead stomata to swiftly close, resulting in an enlarged internal resistance and a reduction in stomatal transpiration. The opening of stomata is controlled by guard cells whose activity is physiologically regulated, whereas that of cuticle transpiration is not (Cernusak et al. 2019; Chen et al. 2020). Strong winds can increase the cuticle transpiration rate, which is enough to offset the reduction of transpiration caused by the closure of stomata, so that less stomatal conductance does not necessarily imply a reduction in the transpiration rate of plants (Burkhardt et al. 1999; England and Attiwill 2011). Therefore, it is possible that when exposed to wind, especially sand-free wind, H. ammodendron seedlings can maintain relatively constant water content in their green assimilative branches; however, sustained sediment-carrying wind often generates a water imbalance caused by more extensive injury to the epidermis of tender branches and augmented cuticle transpiration, resulting in withering and physiological drought in their photosynthetic cells.
4.2 Sustained sediment-carrying wind results in irreversible inactivation of the PSII reaction center
It is generally understood that the harm of sand-free wind and sediment-carrying wind to plants mainly arises via mechanical damage (Anten et al. 2010). Although some studies have investigated the effects of strong wind on plants and their physiological response and comprehensive adaptation to wind stress (e.g., Retuerto and Woodward 1993), few have examined the physiological response of plants after a sandstorm.
The light response curve provides detailed eco-physiological information on the photosynthetic performance of plants as a response to their physiological condition (Brestic and Zivcak 2013; Pleban et al. 2020). Our study indicated that the photochemical efficiency of the PSII reaction center was affected moderately, even in the sediment-carrying wind treatment group (Fig. 2). We assumed that neither wind nor wind-blown sand was able to destroy the integrity of the photosynthetic apparatus of green assimilative branches of H. ammodendron seedlings. However, the light response curves of NPQ were obviously influenced by the blowing of wind-only and sediment-carrying winds (Fig. 1), and their effects were more serious with prolonged wind erosion, suggesting a reduction in the PSII non-photochemical energy dissipation capacity.
Under steady-state PPFD, although a remarkable difference was discernible between the wind-only and sediment-carrying wind treatments, the values of each PSII photochemical efficiency parameter were still very close, which confirmed the results of our rapid light response measurements. Furthermore, the similar values of Fv′/Fm′ at low and high PPFDs and their consistency between wind-only and sediment-carrying wind treatment groups also corroborated the patterns found in the light response curves. Taken together, these results indicated that the integrity of the photosynthetic apparatus was not substantively influenced by either the sand-free wind or sediment-carrying wind within one hour of beginning the chlorophyll fluorescence measurements. Similar to the rapid light curves, NPQ showed consistent variation under steady-state low and high PPFDs. These results verified that with ongoing wind erosion, the non-photochemical quenching ability of the PSII reaction center gradually weakened, and the possibility or ability to avoid photoinhibition and even photodamage in the photosynthetic apparatus likely diminished in tandem. It is clearly evident that the NPQ was sensitive to the blowing of sand-free wind and sediment-carrying wind, although aggravated by the adversity of wind-blown sand. The variation of NPQ involves O2-dependent electron flow, proton gradient across thylakoid membrane (ΔpH), lutein cycle, and inactivation of PSII reaction center (Baker 2008), but its mechanism responding to wind erosion stress needs to be further explored.
After irradiation for a total of 30 min with alternating low and high steady-state light, the dark relaxation was determined; the recovery of the maximum quantum efficiency of PSII photochemistry was measured every 5 min over a 15-min period and denoted Fvr/Fmr. We found no difference in Fvr/Fmr between the two types of wind erosion when blown for 10 min (Fig. 6); however, the wind-blown sand exerted a profound effect on photosynthetic functioning, and the activity of the PSII reaction center did not attain a stable recovery with an extension of the dark relaxation time like that found in the wind-only group. This result is the same as the response of the alpine plant Kobresia humilis after it incurred serious soil drought (Shi et al. 2015). Fvr/Fmr had a similar trend at each dark relaxation time, which matched well with the relative variation in Fv/Fm (Fig. 5). Although the sediment-carrying wind affected Fv/Fm, no pronounced variation within the wind-only group was observed; moreover, short exposure duration to wind-blown sand seemed to not seriously impact the photosynthetic apparatus. The quick recovery of Fvr/Fmr and no serious reduction in Fv/Fm indicated that the functioning of photosynthesis was largely intact and unimpaired (Pilarska et al. 2020) when the wind-blown sand lasted for only 10 min. It was clear that the harmful effects of a sandstorm on the photosynthetic apparatus of H. ammodendron seedlings only appeared if the threshold duration was reached and crossed.
4.3 Instability of photosynthetic functioning indicated a potential harmful impact on photosynthetic function
Compared with the wind-only group, the wind-blown sand was more likely to lessen the photochemical efficiency of the PSII reaction center and drive a decline in the PSII non-radiative energy dissipation of H. ammodendron seedlings; at the same time, the sample standard deviations reflecting the variation degree of each parameter were also greater in the sediment-carrying wind group.
A sample’s standard deviation is an important statistic for determining the extent of variation in the distribution of a sample variable, but its use is not always appropriate when the mean of each sample exhibits a large difference. Therefore, in Table 2, we compared the CVs of Fq′/Fm′, Fq′/Fv′, Fv′/Fm′ and NPQ after irradiation under high steady-state light (at 1500 μmol photons m−2 s−1) and the CV of Fv/Fm after dark adaptation for 15 min. These results uncovered no consistent trends in the CV of each fluorescence parameter with the extension of the wind blowing duration, but the CV values did increase after seedling exposure to sediment-carrying wind. Except for Fq′/Fv′ at 10 min, the CV values of the fluorescence parameters were all higher in the sediment-carrying wind group. Evidently, erosion by wind-blown sand led to an increased instability of photosynthetic function. Further analysis indicated that the maxima and minima of NPQ were relatively lower than those of the wind-only group; a similar pattern also characterized the Fv/Fm (Table 3). Although no exactly coinciding variations of maxima and minima in other parameters exists, the ranges between the maxima and minima were nearly all higher in the sediment-carrying wind group and increased with a longer exposure duration, which was consistent with the trends in CV. Furthermore, except for Fq′/Fv′, the minimum values were lower than those of the wind-only group. These analyses further exemplified that the blowing of sediment-carrying wind exacts a potential harmful impact on the photosynthetic apparatus of H. ammodendron seedlings.
Chlorophyll fluorescence imaging data were obtained within one hour of finishing the simulation treatment in the wind tunnel; the samples were taken from normal assimilative branches, for which no difference was apparent in their epidermal surfaces among different treatments and/or exposure durations except that of 40 min of sediment-carrying wind. Obviously, the emergence of plants’ physiological responses or symptoms usually requires some time, which often lags behind the environmental stimulus, be it a pathogen or an abiotic disturbance factor (De Roo et al. 2020; Chen et al. 2021). A decreased maximum quantum efficiency of PSII photochemistry, Fv/Fm, and its 15-min dark relaxation Fvr/Fmr, as well as a decline in the non-photochemical quenching capacity, all together suggested that inevitable injury have occurred or preexisted in the H. ammodendron seedlings, although notable external morphological or epidermal changes often appeared only several hours later and/or even 1 or 2 days later.
Plant compensation or overcompensation generally refers to a kind of positive self-regulation ability initiated in plants after their exposure to an external stimulus or injury, the essence of which is plant growth redundancy (Dyer et al. 1991; Masini et al. 2019); this ability is a common response in plants after they suffer from or are relieved from adversity (Gaudet and Keddy 1988). Recently, Gao et al. (2016) considered that the main physiological mechanism underpinning this compensatory effect in plants might be caused by the rapid recovery of PSII supplier–recipient side coordination and overriding its control, as well as an overcompensating increase in the number of reactive centers per unit area. In our experiments, the maximum values of Fq′/Fm′ and Fv′/Fm′ increased successively with continued exposure of seedlings to wind erosion (Table 3), which was slightly higher in the sediment-carrying wind than in the wind-only group. This is most likely due to the rapid recovery of photosynthetic function and could be interpreted as a manifestation of compensation and overcompensation effects of the photosynthetic apparatus after physical stress. Wind erosion could cause a downward shift in the minima of PSII photochemical efficiency and non-photochemical energy dissipation, but their maxima sometimes slightly increased. The phenomena of remaining unchanged or slightly increasing in the maximum value of each fluorescence parameter pointed to the existence of compensation or overcompensation effects except for the state of injury. Conversely, a reduction of their minimum values under adversity indicated that more extensive damage was incurred, which is understandable and non-contradictory. Although the average values of fluorescence parameters were not obviously decreased after exposing seedlings to wind erosion adversity, some or clusters of assimilative branches were still in a state of being severely damaged after one night of recovery, especially after exposure to 40 min of sediment-carrying wind. Therefore, we believe that the irreversible damage to the photosynthetic apparatus caused by sandstorms is a key reason for the limited self-renewal of artificial shrubs in arid and semiarid areas; instead, plant competition for water resources may be only one factor leading to population degradation or shrinkage of H. ammodendron plantations.
In conclusion, 12 m s−1 strong wind-blown sand for 10 min did not produce any perceptible injury to the photosynthetic function of H. ammodendron seedlings. In contrast, the Fv/Fm clearly diminished when the winds lasted for 20 min and 40 min, and its recovery of dark relaxation was also restricted. The non-radiative energy dissipation process was sensitive to both sand-free wind and sediment-carrying wind; a decline in NPQ could be accelerated by erosion from wind-blown sand. Incurring longer wind erosion, the NPQ value decreased gradually, indicating the possibility of causing photoinhibition, and even photodamage in the photosynthetic apparatus also increased gradually. Although the water content of seedlings could be maintained under wind blowing treatments, continuous exposure to wind with sand would lead to a water imbalance, which could easily cause wilting and irreversible damage. The blowing of sediment-carrying wind for 40 min often led to the death of branches and even whole plants, mainly due to the sustained hitting of sand grains against the epidermis of green assimilative branches. Therefore, the concentration of photosynthetic pigments based on the fresh mass unit of branches were unaffected; however, the photosynthetic pigment concentration was slightly increased after exposure to sediment-carrying wind erosion, but this was due to an illusion caused by water losses. Irrespective of the type of wind erosion, wind-only or sediment-carrying wind, the photosynthetic apparatus displayed a compensating or overcompensating effect and tended to aggravate the damage incurred. These were the reasons for the increased instability of photosynthetic functioning, which also promoted the PSII photochemical efficiency to present an occasionally high level after wind erosion.
Therefore, the decrease in Fv/Fm and limitation of dark relaxation recovery, as well as the sharp decline in NPQ, are consistent and evidence of an inevitable impact on photosynthetic functioning of H. ammodendron seedlings. The reduction in the minimum value of Fv/Fm and NPQ and the greater likelihood and frequency of lignified spots on the epidermis of green assimilative branches further confirm this inevitable damage. The water imbalance that arose under exposure to continuous sediment-carrying wind affects the activity and vitality of the photosynthetic apparatus, inducing photoinhibition and even photodamage. All these lines of evidence demonstrated that strong and frequent sandstorm events in early spring could lead to irreversible damage to the photosynthetic apparatus in seedlings of H. ammodendron, making it a key factor impeding the self-renewal of artificial shrubs and leading to the shrinkage of its population, so artificial replenishment of seedlings is a necessary measure for adult shelter forest management.
Data availability
Data will be made available if requested to the corresponding author.
References
Anten NPR, Alcalá-Herrera R, Schieving F, Onoda Y (2010) Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytol 188(2):554–564. https://doi.org/10.1111/j.1469-8137.2010.03379.x
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker NR, Rosenqvist E (2004) Application of chlorophyll fluorescence and improve crop production strategies: an examination of future possibilities. J Exp Bot 55(403):1607–1621. https://doi.org/10.1093/jxb/erh196
Bedunah DJ, Schmidt SM (2006) Rangelands of gobi gurvan saikham natural conservation park, Mongolia. Rangelands 22:18–24. https://doi.org/10.2307/4001540
Bilger W, Björkman O (1990) Role of the xanthophyll cycle photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25(3):173–185. https://doi.org/10.1007/BF00033159
Bormann FH, Likens GE (1981) Pattern and process in a forested ecosystem. Springer-Verlag, Heidelberg
Brestic M, Zivcak M (2013) PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and Applications. In: Rout GR, Das AB (eds) Molecular Stress Physiology of Plants. Springer, Dordrecht Heidelberg, New York, London, pp 87–132
Burkhardt J, Kaiser H, Goldbach H, Kappen L (1999) Measurements of electrical leaf surface conductance reveal re-condensation of transpired water vapour on leaf surfaces. Plant Cell Environ 22(2):189–196
Cernusak LA, Goldsmith GR, Arend M, Siegwolfb RTW (2019) Effect of vapor pressure deficit on gas exchange in wild-type and abscisic acid-insensitive plants. Plant Physiol 181(4):1573–1586
Chen MJ, Zhu XF, Zhang Y, Du ZH, Chen XB, Kong XR, Sun WJ, Chen CS (2020) Drought stress modify cuticle of tender tea leaf and mature leaf for transpiration barrier enhancement through common and distinct modes. Sci Rep-UK 10(1):6696. https://doi.org/10.1038/S41598-020-63683-4
Chen WY, Jia B, Chen JY, Feng YJ, Li Y, Chen MT, Liu HH, Yin ZT (2021) Effects of different planting densities on photosynthesis in maize determined via prompt fluorescence, delayed fluorescence and P700 signals. Plants 10(2):276–283
De Roo L, Salomón RL, Oleksyn J, Steppe K (2020) Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytol 228(1):70–81
Dong ZB, Chen GT, He XD, Han ZW, Wang XM (2004) Controlling blown sand along the highway crossing the Taklimakan Desert. J Arid Environ 57(3):329–344. https://doi.org/10.1016/j.jaridenv.2002.02.001
Dyer MI, Turner CL, Seastedt TR (1991) Mowing and fertilization effect on productivity and spectral reflectance in Bromus inermis plots. Ecol Appl 1:443–452. https://doi.org/10.2307/1941901
Edelfeldt S, Bengtsson K, Dahlgren JP (2019) Demographic senescence and effects on population dynamics of a perennial plant. Ecology 100(8):e02742. https://doi.org/10.1002/ecy.2742
England JR, Attiwill PM (2011) Changes in stomatal frequency, stomatal conductance and cuticle thickness during leaf expansion in the broad-leaved evergreen species Eucalyptus regnans. Trees 25(6):987–996
Gao J, Li QF, Xue JQ, Zhang RH (2016) Physiological compensation mechanism of photosystem II in maize leaves induced by drought stress and re-watering condition. Plant Physiol J 52(9):1413–1420
Gaudet CI, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334(6179):242–243. https://doi.org/10.1038/334242a0
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen Subjects 990(1):87–92. https://doi.org/10.1016/s0304-4165(89)80016-9
Han JD, Zhang H, Ni ZY, Yao ZP, Wang Z, Ren C, Chen QJ, Ma H (2016) Cloning and transcriptional activation analysis of NAC gene family from Haloxylon ammodendron. Genom Appl Biol 35(11):3163–3171
He FL, Jin HX, Guo CX, Ma JM, Wu H (2017) Vegetation composition and community similarity of Haloxylon ammodendron plantation at different degree of degradation on the edge of the Minqin oasis. J Desert Res 27(6):1135–1141
Henry HAL, Thomas SC (2002) Interactive effects of lateral shade and wind on stem allometry, biomass allocation, and mechanical stability in Abutilon theophrasti (Malvaceae). Am J Bot 89(10):1609–1615
Jiang JF, Liang CH, Yang H, Zhang YL, Ding WK, Yang YL (2017) Influence of temperature and precipitation on phenology of desert plant Haloxylon ammodendron and Cornulaca laschanica in recent ten years. J Arid Land Resour Environ 31(2):141–146
Ju Q, Gong L, Yang JL, Pan XL (2005) Interrelation between the process of photosynthetic physiological ecology of Haloxylon ammodendron and xeric environment. J Arid Land Resour Environ 19(4):201–204
Li ZS, Ni JR (1998) Aeolian sand transport processed. J Arid Land Resour Environ 12(3):89–97. https://doi.org/10.13448/j.cnki.jalre.1998.03.015(ChineseVersion)
Li DM, Ji YF, Zhang YH, Wei LY, Wang F, Ma R, Jiang ZR (2019) Effects of wind-drift sand on physiological characteristics of Zygophyllum xanthoxylum seedling. J Northwest a&f Univ 47(3):103–110
Masini L, Grenville-Briggs LJ, Andreasson E, Råberg L, Lankinen Å (2019) Tolerance and overcompensation to infection by Phytophthora infestans in the wild perennial climber Solanum dulcamara. Ecol Evol 9(8):4557–4567. https://doi.org/10.1002/ece3.5057
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51(345):659–668. https://doi.org/10.1093/jxb/51.345.659
Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16(3):307–314. https://doi.org/10.1016/j.pbi.2013.03.011
Ogunjobiet KO, Kim YJ, He Z (2003) Aerosol optical properties during Asian dust storm episodes in South Korea. Theor Appl Climatol 76(1–2):65–75. https://doi.org/10.1007/s00704-003-0006-7
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth Res 54(2):135–142. https://doi.org/10.1023/A:1005936823310
Pilarska M, Niewiadomska E, Sychta K, Słomkal A (2020) Differences in the functioning of photosynthetic electron transport between metallicolous and non-metallicolous populations of the pseudometallophyte Viola tricolor. J Plant Physiol. https://doi.org/10.1016/j.jplph.2020.153185
Pleban JR, Guadagno CR, Mackay DS, Weinig C, Ewers BE (2020) Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol (bethesda) 183(2):602–619. https://doi.org/10.1104/pp.19.00375
Qu H, Zhao XY, Yue GY, Wang SK (2009) Physiological response to wind of some common plants in Horqin Sandy Land. J Desert Res 29(4):668–673
Qu JJ, Ling YQ, Liu BJ, Chen GT, Wang T, Dong ZB (2019) Research status and development trends of wind-sand engineering in China. Adv Earth Sci 34(3):225–231. https://doi.org/10.11867/j.issn.1001-8166.2019.03.0225(ChineseVersion)
Retuerto R, Woodward FI (1993) The influences of increased CO2 and supply on growth, biomass allocation and water use efficiency of Sinapis alba L. grown under different wind speeds. Oecologia 94(3):415–427
Shi PJ, Yan P, Gao SY, Wang YM, Ha S, Yu YJ (2000) The dust storm disaster in China and its research progress. J Nat Disasters 9(3):71–77. https://doi.org/10.3969/j.issn.1004-4574.2000.03.011(ChineseVersion)
Shi SB, Zhu WY, Li HM, Zhou DW, Han F, Zhao XQ, Tang YH (2004) Photosynthesis of Saussurea superba and Gentiana straminea is not reduced after long-term enhancement of UV-B radiation. Environ Exp Bot 51(1):75–83
Shi SB, Li TC, Li M, Liu SZ, Li AD, Ma JP (2015) Interaction effect analysis of soil-drought and strong light on in Kobresia pygmaea leaves. Plant Physiol J 51:1678–1686. https://doi.org/10.1007/S11738-015-1987-4
Si LM, Liu T, Liu B, Li L (2011) A comparative study on reasons of degenerated of Haloxylon ammodendron population in the western part of Gurbantunggut desert. Acta Ecol Sin 31(21):6460–6468. https://doi.org/10.7666/d.d178187(ChineseVersion)
Sun T, Ma JP, Shi SB, Han FG, Zhang YN, Wang FL, Wan X, Zhang HY (2021) Effect on the phenotype of Haloxylon ammodendron by wind sand-blown stress simulated in the wind tunnel. For Environ Sci 34(7):7–14
Tian XM, Zhao CM, Deng JM, Zhang XW, Chen T, Ren JW, Wang GX (2011) Photosynthetic responses of four dominant species to environmental gradient along the oasis–desert ecotone of Minqin, China. Acta Prataculture Sinica 20(4):108–115
Tobe K, Li XM, Omasa K (2000) Effect of sodium chloride on seed germination and growth of two Chinese desert shrubs Haloxylon ammodendron and H. persicum. Aust J Bot 48(4):455–460
Wang JH, Ma QL (2003) Study on restoration strategies, characteristics and status of degenerated artificial Haloxylon ammodendron communities at the edge of Minqin oasis. Acta Botan Boreali-Occiden Sin 23(12):2107–2112. https://doi.org/10.3321/j.issn:1000-4025.2003.12.010(ChineseVersion)
Wang GH, Zhao WZ (2015) Effects of seed density on the germination and seedling growth of Haloxylon ammodendron. J Desert Res 35(5):1248–1253
Wang T, Chen GT, Zhao HL, Dong ZB, Zhang XY, Zheng XJ, Wang NA (2006) Research progress on aeolian desertification process and controlling in north of China. J Desert Res 26(4):507–516. https://doi.org/10.3321/j.issn:1000-694X.2006.04.001
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol 144(3):307–313
Yu JY, Shi PJ, He LP, Liu JQ (2002) Research on the effects of windsand current on the plant growth. Adv Earth Sci 17(2):262–267. https://doi.org/10.3321/j.issn:1001-8166.2002.02.016
Zhan KJ, Zhao M, Fang ET, Yang ZH, Zhang YC, Guo SJ, Zhang JC, Wang QQ, Wang DZ (2009) The wind speed characteristics of near-surface vertical gradient of 50 m in sandstorm process in 2006. J Arid Land Resour Environ 23(9):100–105
Zhang JC, Wang JH, An FB, Sun T, Liu YJ, Li YK, Xiao B (2009) Population CHARACTERISTICS of Natural Haloxylon ammoderon in Minqin. Gansu of China. J Desert Res 29(6):1124–1128
Zhang KC, Qu JJ, Liao KT, Niu QH, Han QJ (2010) Damage by wind-blown sand and its control along Qinghai-Tibet Railway in China. Aeolian Res 1(3–4):143–146. https://doi.org/10.1016/j.aeolia.2009.10.001
Zhao SX, Sun JH (2013) Study on the mechanism and prediction of disastrous weathers during recent years. Chin J Atmos Sci 37(2):297–312. https://doi.org/10.3878/j.issn.1006-9895.2012.12317
Zhao HL, Li J, Zhou RL, Yun JY, Qu H, Pan CC (2015) Effects of strong wind-drift blowing on the growth and physiological properties of Pinus sylvestris var. mongolica seedlings. Chin J Ecol 34(4):901–906
Zhao HL, Li J, Zhou RL, Yun JY, Feng J, Sun N (2017) Effects of brief sand-drift wind blowing on photosynthesis and transpiration properties of Pinus sylvestris var. mogolica seedling. J Desert Res 37(2):254–260. https://doi.org/10.7522/j.issn.1000-694X.2015.00191
Acknowledgements
We would like to thank Profs. Cai-zhou Kang and Yong-fu Ji at the Gansu Desert Control Research Institute for kindly providing help with the daily field management and the statistical analysis of the data. This work was financially supported by the National Natural Science Foundation of China (31660237), the Qinghai Province Science Foundation (2019-ZJ-7016), and the Construction Project for Innovation Platform of Qinghai Province (2017-ZJ-Y20; 2017-ZJ-Y14).
Author information
Authors and Affiliations
Contributions
SBS presided the project’s execution, conceived the study, designed the experimental protocols, analyzed the data, and drafted the manuscript. DWZ and FLW helped to design the experimental protocols, arranged for specific experiments and revised the manuscript. RS conceived the study, analyzed the data, and wrote the first draft of the manuscript. TCL, TS, JLM and XW carried out the experiments and collected data. JPM and JNT contributed to the experimental design and participated in discussions on the manuscript’s text and writing. All authors contributed to the study conception, and design, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors are no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Sb., Zhou, Dw., Wang, Fl. et al. Sandstorms cause shrinkage of Haloxylon ammodendron shrubs and limit their self-renewal. Theor. Exp. Plant Physiol. 34, 197–214 (2022). https://doi.org/10.1007/s40626-022-00242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-022-00242-4