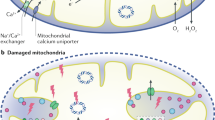
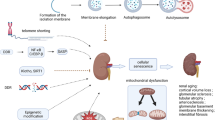
Abstract
Acute kidney disease (AKD) involves multiple pathogenic mechanisms, including maladaptive repair of renal cells that are rich in mitochondria. Maintenance of mitochondrial homeostasis and quality control is crucial for normal kidney function. Mitochondrial quality control serves to maintain mitochondrial function under various conditions, including mitochondrial bioenergetics, mitochondrial biogenesis, mitochondrial dynamics (fusion and fission) and mitophagy. To date, increasing evidence indicates that mitochondrial quality control is disrupted when acute kidney disease develops. This review describes the mechanisms of mitochondria quality control in acute kidney disease, aiming to provide clues to help design new clinical treatments.


Similar content being viewed by others
References
Kellum JA et al (2012) Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. Kdigo clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. https://doi.org/10.1038/kisup.2012.1
Levin A et al (2013) Kidney Disease: Improving Global Outcomes (KDIGO). Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150. https://doi.org/10.1038/kisup.2012.73
Levey AS (2021) Defining AKD: the spectrum of AKI, AKD, and CKD. Nephron. https://doi.org/10.1159/000516647
Funk JA, Schnellmann RG (2012) Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Ren Physiol 302(7):F853–F864. https://doi.org/10.1152/ajprenal.00035.2011
Aparicio-Trejo OE et al (2020) Chronic impairment of mitochondrial bioenergetics and β-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Rad Biol Med 154:18–32. https://doi.org/10.1016/j.freeradbiomed.2020.04.016
Duann P, Lin PH (2017) Mitochondria damage and kidney disease. Adv Exp Med Biol 982:529–551. https://doi.org/10.1007/978-3-319-55330-6_27
Bhargava P, Schnellmann RG (2017) Mitochondrial energetics in the kidney. Nat Rev Nephrol 13(10):629–646. https://doi.org/10.1038/nrneph.2017.107
Chawla LS et al (2017) Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 13(4):241–257. https://doi.org/10.1038/nrneph.2017.2
Levey AS et al (2020) Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 97(6):1117–1129. https://doi.org/10.1016/j.kint.2020.02.010
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet (Lond Engl). 394(10212):1949–1964. https://doi.org/10.1016/S0140-6736(19)32563-2
Kellum JA et al (2017) Recovery after acute kidney injury. Am J Respir Crit Care Med 195(6):784–791. https://doi.org/10.1164/rccm.201604-0799OC
Shah S et al (2020) Mortality and recovery associated with kidney failure due to acute kidney injury. Clin J Am Soc Nephrol CJASN. https://doi.org/10.2215/CJN.11200919
Yan P et al (2021) Acute kidney disease in hospitalized acute kidney injury patients. PeerJ 9:e11400. https://doi.org/10.7717/peerj.11400
Venkatachalam MA et al (2015) Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26(8):1765–1776. https://doi.org/10.1681/ASN.2015010006
Basile DP et al (2016) Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol JASN 27(3):687–697. https://doi.org/10.1681/ASN.2015030309
Bomsztyk K, Denisenko O (2013) Epigenetic alterations in acute kidney injury. Semin Nephrol 33(4):327–340. https://doi.org/10.1016/j.semnephrol.2013.05.005
Docherty M-H et al (2019) Cellular senescence in the kidney. J Am Soc Nephrol JASN 30(5):726–736. https://doi.org/10.1681/ASN.2018121251
Ferenbach DA, Bonventre JV (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11(5):264–276. https://doi.org/10.1038/nrneph.2015.3
Tang C et al (2021) Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol 17(5):299–318. https://doi.org/10.1038/s41581-020-00369-0
Singh AP et al (2020) Molecular connectivity of mitochondrial gene expression and OXPHOS biogenesis. Molecular Cell. https://doi.org/10.1016/j.molcel.2020.07.024
Ratliff BB et al (2016) Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25(3):119–146. https://doi.org/10.1089/ars.2016.6665
Coppolino G et al (2018) Oxidative stress and kidney function: a brief update. Curr Pharmaceut Design 24(40):4794–4799. https://doi.org/10.2174/1381612825666190112165206
Mapuskar KA et al (2019) Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. https://doi.org/10.1016/j.redox.2018.09.020
Kim J et al (2009) Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Ren Physiol 297(2):F461–F470. https://doi.org/10.1152/ajprenal.90735.2008
Szeto HH (2014) First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171(8):2029–2050. https://doi.org/10.1111/bph.12461
Zhang X, Agborbesong E, Li X (2021) The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci. https://doi.org/10.3390/ijms222011253
Ding W et al (2016) Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget 7(14):17479–17491. https://doi.org/10.18632/oncotarget.8243
Popov L-D (2020) Mitochondrial biogenesis: an update. J Cell Mol Med 24(9):4892–4899. https://doi.org/10.1111/jcmm.15194
Jamwal S, Blackburn JK, Elsworth JD (2021) PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol Thera 219:107705. https://doi.org/10.1016/j.pharmthera.2020.107705
Chambers JM, Wingert RA (2020) PGC-1α in disease: recent renal insights into a versatile metabolic regulator. Cells. https://doi.org/10.3390/cells9102234
Tran M et al (2011) PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121(10):4003–4014. https://doi.org/10.1172/JCI58662
Portilla D et al (2002) Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int 62(4):1208–1218
Cherry AD et al (2014) Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J Biol Chem 289(1):41–52. https://doi.org/10.1074/jbc.M113.512483
Fontecha-Barriuso M et al (2020) The role of PGC-1α and mitochondrial biogenesis in kidney diseases. Biomolecules. https://doi.org/10.3390/biom10020347
Kelly DP, Scarpulla RC (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18(4):357–368
Yuan Y et al (2012) Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82(7):771–789. https://doi.org/10.1038/ki.2012.188
Platt C, Coward RJ (2017) Peroxisome proliferator activating receptor-γ and the podocyte. Nephrol Dial Transp 32(3):423–433. https://doi.org/10.1093/ndt/gfw320
Collier JB, Schnellmann RG (2020) Extracellular signal-regulated kinase 1/2 regulates NAD metabolism during acute kidney injury through microRNA-34a-mediated NAMPT expression. Cell Mol Life Sci CMLS. 77(18):3643–3655. https://doi.org/10.1007/s00018-019-03391-z
Gibbs WS et al (2018) 5-HT receptor regulates mitochondrial homeostasis and its loss potentiates acute kidney injury and impairs renal recovery. Am J Physiol Ren Physiol 315(4):F1119–F1128. https://doi.org/10.1152/ajprenal.00077.2018
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884S – 8890. https://doi.org/10.3945/ajcn.110.001917
Stallons LJ, Whitaker RM, Schnellmann RG (2014) Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol Lett 224(3):326–332. https://doi.org/10.1016/j.toxlet.2013.11.014
Jiang M et al (2019) Combined blockade of Smad3 and JNK pathways ameliorates progressive fibrosis in folic acid nephropathy. Front Pharmacol. 10:880. https://doi.org/10.3389/fphar.2019.00880
Chan DC (2020) Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol 15:235–259. https://doi.org/10.1146/annurev-pathmechdis-012419-032711
Zhan M et al (2013) Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83(4):568–581. https://doi.org/10.1038/ki.2012.441
Tilokani L et al (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62(3):341–360. https://doi.org/10.1042/EBC20170104
Tang C et al (2018) PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy. 14(5):880–897. https://doi.org/10.1080/15548627.2017.1405880
Lei R et al (2018) Mitophagy plays a protective role in iodinated contrast-induced acute renal tubular epithelial cells injury. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 46(3):975–985. https://doi.org/10.1159/000488827
Wang Y et al (2020) Mitophagy in acute kidney injury and kidney repair. Cells. https://doi.org/10.3390/cells9020338
Yan Y et al (2020) miR-214 represses mitofusin-2 to promote renal tubular apoptosis in ischemic acute kidney injury. Am J Physiol Ren Physiol 318(4):F878–F887. https://doi.org/10.1152/ajprenal.00567.2019
Wang Y et al (2020) Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis 11(1):29. https://doi.org/10.1038/s41419-019-2218-5
Gall JM et al (2015) Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol JASN. 26(5):1092–1102. https://doi.org/10.1681/ASN.2014010126
Livingston MJ et al (2019) Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15(12):2142–2162. https://doi.org/10.1080/15548627.2019.1615822
Lin Q et al (2019) PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 26:101254. https://doi.org/10.1016/j.redox.2019.101254
Inoue T, Maekawa H, Inagi R (2019) Organelle crosstalk in the kidney. Kidney Int 95(6):1318–1325. https://doi.org/10.1016/j.kint.2018.11.035
Senft D, Ronai ZEA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 40(3):141–148. https://doi.org/10.1016/j.tibs.2015.01.002
Lombardi AA, Elrod JW (2017) Mediating ER-mitochondrial cross-talk. Science (New York NY). 358(6363):591–592. https://doi.org/10.1126/science.aaq0141
Maekawa H, Inagi R (2019) Pathophysiological role of organelle stress/crosstalk in AKI-to-CKD. Trans Semin Nephrol 39(6):581–588. https://doi.org/10.1016/j.semnephrol.2019.10.007
Kezic A et al (2016) Mitochondria-targeted antioxidants: future perspectives in kidney ischemia reperfusion injury. Oxid Med Cell Long 2016:2950503. https://doi.org/10.1155/2016/2950503
Dare AJ et al (2015) Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant. MitoQ Redox Biol 5:163–168. https://doi.org/10.1016/j.redox.2015.04.008
Song J et al (2022) Mitochondrial targeted antioxidant SKQ1 ameliorates acute kidney injury by inhibiting ferroptosis. Oxid Med Cell Longev 2022:1–19. https://doi.org/10.1155/2022/2223957
Plotnikov EY et al (2011) Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochimica et Biophysica Acta 1812(1):77–86. https://doi.org/10.1016/j.bbadis.2010.09.008
Mukhopadhyay P et al (2012) Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radical Biol Med 52(2):497–506. https://doi.org/10.1016/j.freeradbiomed.2011.11.001
Birk AV et al (2013) The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol JASN. 24(8):1250–1261. https://doi.org/10.1681/ASN.2012121216
Szeto HH et al (2017) Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1 and IL-18 and arrests CKD. J Am Soc Nephrol JASN. 28(5):1437–1449. https://doi.org/10.1681/ASN.2016070761
Liu S et al (2014) Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Ren Physiol 306(9):F970–F980. https://doi.org/10.1152/ajprenal.00697.2013
Szeto HH et al (2015) Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Ren Physiol 308(1):F11–F21. https://doi.org/10.1152/ajprenal.00366.2014
Tran MT et al (2016) PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 531(7595):528–532. https://doi.org/10.1038/nature17184
Lempiäinen J et al (2012) AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 166(6):1905–1915. https://doi.org/10.1111/j.1476-5381.2012.01895.x
Shin YJ et al (2015) Protective effects of quercetin against HgCl2-induced nephrotoxicity in sprague-dawley rats. J Med Food. 18(5):524–534. https://doi.org/10.1089/jmf.2014.3242
Kitada M, Koya D (2013) Renal protective effects of resveratrol. Oxidat Med Cell Long. 2013:568093. https://doi.org/10.1155/2013/568093
Funk JA, Schnellmann RG (2013) Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 273(2):345–354. https://doi.org/10.1016/j.taap.2013.09.026
Jesinkey SR et al (2014) Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol JASN 25(6):1157–1162. https://doi.org/10.1681/ASN.2013090952
Cameron RB et al (2019) Proximal tubule -adrenergic receptor mediates formoterol-induced recovery of mitochondrial and renal function after ischemia-reperfusion injury. J Pharmacol Exp Thera 369(1):173–180. https://doi.org/10.1124/jpet.118.252833
Garrett SM et al (2014) Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Thera 350(2):257–264. https://doi.org/10.1124/jpet.114.214700
Chen W et al (2018) Pioglitazone protects against renal ischemia-reperfusion injury via the AMP-activated protein kinase-regulated autophagy pathway. Front Pharmacol 9:851. https://doi.org/10.3389/fphar.2018.00851
Suzuki T et al (2016) Mitochonic Acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J Am Soc Nephrol JASN. 27(7):1925–1932. https://doi.org/10.1681/ASN.2015060623
Poyan Mehr A et al (2018) De novo NAD biosynthetic impairment in acute kidney injury in humans. Nat Med 24(9):1351–1359. https://doi.org/10.1038/s41591-018-0138-z
Katsyuba E et al (2018) De novo NAD synthesis enhances mitochondrial function and improves health. Nature 563(7731):354–359. https://doi.org/10.1038/s41586-018-0645-6
Bouchez S et al (2018) Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Therapy 32(6):617–624. https://doi.org/10.1007/s10557-018-6838-2
Cassidy-Stone A et al (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14(2):193–204. https://doi.org/10.1016/j.devcel.2007.11.019
Brooks C et al (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Investig 119(5):1275–1285. https://doi.org/10.1172/JCI37829
Rosdah AA et al (2016) Mitochondrial fission—a drug target for cytoprotection or cytodestruction? Pharmacol Res Perspect. 4(3):e00235. https://doi.org/10.1002/prp2.235
Tang W-X et al (2013) Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol. 26(6):1073–1082. https://doi.org/10.5301/jn.5000268
Perry HM et al (2018) Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol JASN. 29(1):194–206. https://doi.org/10.1681/ASN.2017060659
Bordt EA et al (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex i inhibitor that modulates reactive oxygen species. Dev Cell. https://doi.org/10.1016/j.devcel.2017.02.020
Cui J et al (2015) Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep 5:11256. https://doi.org/10.1038/srep11256
Livingston MJ et al (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12(6):976–998. https://doi.org/10.1080/15548627.2016.1166317
Zou D et al (2019) Oral delivery of nanoparticle urolithin A normalizes cellular stress and improves survival in mouse model of cisplatin-induced AKI American journal of physiology. Ren Physiol 317(5):F1255–F1264. https://doi.org/10.1152/ajprenal.00346.2019
Wang Z et al (2013) Redox-sensitive glycogen synthase kinase 3β-directed control of mitochondrial permeability transition: rheostatic regulation of acute kidney injury. Free Radical Biol Med 65:849–858. https://doi.org/10.1016/j.freeradbiomed.2013.08.169
Wang Z et al (2015) Pharmacological targeting of GSK3β confers protection against podocytopathy and proteinuria by desensitizing mitochondrial permeability transition. Br J Pharmacol. 172(3):895–909. https://doi.org/10.1111/bph.12952
Zhao K et al (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279(33):34682–34690
Zhang W et al (2018) Rotenone ameliorates chronic renal injury caused by acute ischemia/reperfusion. Oncotarget 9(36):24199–24208. https://doi.org/10.18632/oncotarget.24733
Aparicio-Trejo OE et al (2017) Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: relation to oxidative stress and mitochondrial bioenergetics. BioFactors (Oxf, Engl) 43(2):293–310. https://doi.org/10.1002/biof.1338
Funding
This work was supported by the National Natural Science Foundation of China (grant. no. 82100719); the Natural Science Foundation of Jiangsu Province (Grant. no. BK20210982).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, J., Li, X., Lei, J. et al. Mitochondrial quality control in acute kidney disease. J Nephrol 36, 1283–1291 (2023). https://doi.org/10.1007/s40620-023-01582-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01582-3