Abstract
Introduction
Eculizumab is effective treatment of pediatric atypical hemolytic uremic syndrome (aHUS). However, the optimal duration of treatment is not clearly defined. The aim of this study was to retrospectively analyze the outcome of pediatric patients with aHUS, who started eculizumab treatment but discontinued it during the follow-up period.
Methods
The clinical and laboratory findings of the pediatric patients with aHUS were recorded on a web-based, national registry system, known as the Turkish aHUS Registry. The study included 63 patients who had to have received more than four doses of eculizumab during the acute phase of the disease.
Results
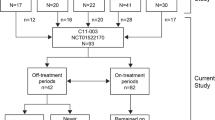
The median age at diagnosis was 3.62 (IQR: 1.29–6.14) years. During the follow-up period, 39 patients continued to receive standard eculizumab treatment (standard treatment group, treatment every 2 weeks) while 24 received an extended dose of eculizumab at three-four-week intervals (non-standard treatment group). There was no significant difference between both groups in terms of clinical and laboratory parameters. Eculizumab treatment was discontinued in 18 patients (30.7%, F/M:11/7), and the median age of these patients at diagnosis and their median follow-up duration were 4.0 (IQR:2.7–10.2) and 4.2 (IQR:2.2–7) years respectively. The median eGFR at the last visit was 110 (84.7–146.1)ml/min/1.73 m2. Fourteen patients remained in remission without any sign of the disease. Recurrence occurred in four (22.2%) patients, in which eculizumab was immediately started again and complete remission was achieved.
Conclusion
Eculizumab is a successful treatment option in pediatric patients with aHUS and it can be safely discontinued with close monitoring in a selected group of patients. In case of recurrence, eculizumab should be restarted immediately to achieve complete remission.
Graphical abstract


Similar content being viewed by others
Change history
07 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40620-022-01265-5
References
Loirat C, Fakhouri F, Ariceta G et al (2016) An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 31:15–39. https://doi.org/10.1007/s00467-015-3076-8
Yan K, Desai K, Gullapalli L, Druyts E, Balijepalli C (2020) Epidemiology of atypical hemolytic uremic syndrome. A systematic literature review. Clin Epidemiol 12:295–305. https://doi.org/10.2147/CLEP.S245642
Ozaltin F, Li B, Rauhauser A et al (2013) DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol 24(3):377–384. https://doi.org/10.1681/ASN.2012090903
Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C (2017) Haemolytic uraemic syndrome. Lancet 217:681–696. https://doi.org/10.1016/S0140-6736(17)30062-4
Fremeaux-Bacchi V, Fakhouri F, Garnier A et al (2013) Genetics and outcome of atypical hemolytic uremic syndrome:a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8:554–562. https://doi.org/10.2215/CJN.04760512
Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, GambaS DE, Fenili C, Castelletti F, Sorosina A, Piras R, DonadelliR MR, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G (2010) Relative role of genetic complement abnormalitiesin sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5:1844–1859. https://doi.org/10.2215/CJN.02210310
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T et al (2013) Terminal complement inhibitor eculizumab in atypicalhemolytic-uremic syndrome. N Engl J Med 368:2169–2181. https://doi.org/10.1056/NEJMoa1208981
Greenbaum LA, Fila M, Ardissino G et al (2016) Eculizumab is a safe and effective treatment inpediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711. https://doi.org/10.1016/j.kint.2016.06.006
Tanaka K, Adams B, Aris AM et al (2021) The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab. Pediatr Nephrol 36:889–898. https://doi.org/10.1007/s00467-020-04774-2
Fakhouri F, Fila M, Provot F et al (2017) Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 12:50–59. https://doi.org/10.2215/cjn.06440616
Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O (2015) Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 30(5):783–789. https://doi.org/10.1007/s00467-014-3003-4
Goodship TH, Cook HT, Fakhouri F et al (2017) Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “kidney disease: improving global outcomes” (KDIGO) controversies conference. Kidney Int 91:539–551. https://doi.org/10.1016/j.kint.2016.10.005
Ariceta G (2019) Optimal duration of treatment with eculizumab in atypical hemolytic uremicsyndrome (ahus)-a question to be addressed in a scientific way. Pediatr Nephrol 34:943–949. https://doi.org/10.1007/s00467-019-4192
Fakhouri F, Loirat C (2018) Anticomplement treatment in atypical and typical hemolytic uremic syndrome. Semin Hematol 55:150–158. https://doi.org/10.1053/j.seminhematol
Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA (2017) Eculizumab cessation in atypical hemolytic uremic syndrome. Blood 130:368–372. https://doi.org/10.1182/blood-2017-02-770214
Ardissino G, Testa S, Possenti I et al (2014) Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 64:633–637. https://doi.org/10.1053/j.ajkd.2014.01.434
Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V (2015) Discontinuation of eculizumab treatment in atypical hemolyticuremic syndrome: an update. Am J Kidney Dis 66:172–173. https://doi.org/10.1053/j.ajkd.2015.04.010
Fakhouri F, Fila M, Hummel A et al (2020) Eculizumab discontinuation in children and adults with atypical haemolytic uremic syndrome: a prospective multicentric study. Blood. https://doi.org/10.1182/blood.2020009280
Besbas N, Gulhan B, Soylemezoglu O, Ozcakar ZB et al (2017) Turkish pediatric atypical hemolytic uremic syndrome registry: initial analysis of 146 patients. BMC Nephrol 18(1):6. https://doi.org/10.1186/s12882-016-0420-6
Mian AN, Schwartz GJ (2017) Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis 24(6):348–356. https://doi.org/10.1053/j.ackd.2017.09.011
Saida K, Fukuda T, Mizuno K et al (2019) Pharmacokinetics and pharmacodynamics estimation of eculizumab in a 2-year-old girl with atypical hemolytic uremic syndrome: a case report with 4-year follow-up. Front Pediatr 17(7):519. https://doi.org/10.3389/fped.2019.00519
Gatault P, Brachet G, Ternant D et al (2015) Therapeutic drug monitoring of eculizumab: rationale for an individualized dosing schedule. MAbs 7:1205–1211. https://doi.org/10.1080/19420862.2015.1086049
Volokhina E, Wijnsma K, van der Molen R, Roeleveld N et al (2017) Eculizumab dosing regimen in atypical HUS: possibilities for individualized treatment. Clin Pharmacol Ther 102:671–678. https://doi.org/10.1002/cpt.686
Ardissino G, Tel F, Sgarbanti M et al (2017) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol. https://doi.org/10.1007/s00467-017-3813-2
Puissant-Lubrano B, Puissochet S, Congy-Jolivet N et al (2017) Alternative complement pathway hemolytic assays revealincomplete complement blockade in patients treated with eculizumab. Clin Immunol 183:1–7. https://doi.org/10.1016/j.clim.2017.06.007
Wehling C, Amon O, Bommer M, Hoppe B et al (2017) (2017) Monitoring of complementactivation biomarkers and eculizumab in complement complement mediated renal disorders. Clin Exp Immunol 187:304–315. https://doi.org/10.1111/cei.12890
Macia M, de Alvaro MF, Dutt T et al (2017) Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J 10:310–319. https://doi.org/10.1093/ckj/sfw115
Wijnsma KL, Duineveld C, Volokhina EB et al (2018) Safety and effectiveness of restrictive eculizumab treatment in atypical haemolytic uremic syndrome. Nephrol Dial Transplant 33(4):635–645. https://doi.org/10.1093/ndt/gfx196
Sheerin NS, Kavanagh D, Goodship TH, Johnson S (2016) A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM 109:27–33. https://doi.org/10.1093/qjmed/hcv082
Wijnsma KL, Duineveld C, Wetzels JFM, van de Kar NCAJ (2019) Eculizumab in atypical hemolytic uremic syndrome: strategies toward restrictive use. Pediatr Nephrol 34:2261–2277. https://doi.org/10.1007/s00467-018-4091.32
Menne J, Delmas Y, Fakhouri F et al (2019) Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clin Kidney J 12:196–205. https://doi.org/10.1093/ckj/
Menne J, Delmas Y, Fakhouri F et al (2019) Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol 20:125. https://doi.org/10.1186/s12882-019-1314-1
Ariceta G, Fakhouri F, Sartz L et al (2021) Eculizumab discontinuation in atypical hemolytic uremic syndrome: TMA recurrence risk and renal outcomes. Clin Kidney J. https://doi.org/10.1093/ckj/sfab005
Ozcakar ZB, Ozaltin F, Gülhan B et al (2020) Transplantation in pediatric aHUS within the era of eculizumab therapy. Pediatr Transplant 20:e13914. https://doi.org/10.1111/petr.13914
Funding
This manuscript did not receive any specific grant from funding agencies in the public, private, commercial, or not-for profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical statement
The study was approved by the ethics committee of Hacettepe University (FON10/03-22). The authors confirmed their adherence to the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been updated: Due to co-author family name and surname update.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baskin, E., Fidan, K., Gulhan, B. et al. Eculizumab treatment and discontinuation in pediatric patients with atypical hemolytic uremic syndrome: a multicentric retrospective study. J Nephrol 35, 1213–1222 (2022). https://doi.org/10.1007/s40620-021-01212-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01212-w