Abstract
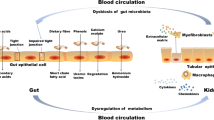
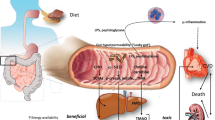
The gut microbiota is considered to be a novel important factor to take into account in the pathogenesis of chronic kidney disease and uremia. Much attention has been paid to specific uremic retention solutes of microbial origin, such as indoxyl sulfate, p-cresyl sulfate, and trimethylamine-N-oxide. However, other novel less well studied compounds, such as hydrogen sulfide and related sulfur metabolites (sulfane sulfur, lanthionine, etc.), should be included in a more comprehensive appraisal of this topic, in light of the potential therapeutic opportunities for the future.



Similar content being viewed by others
References
Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) (2003) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 63(5):1934–1943
Meijers B, Glorieux G, Poesen R, Bakker SJ (2014) Nonextracorporeal methods for decreasing uremic solute concentration: a future way to go? Semin Nephrol 34(2):228–243
Einheber A, Carter D (1966) The role of the microbial flora in uremia. I. Survival times of germfree, limited-flora, and conventionalized rats after bilateral nephrectomy and fasting. J Exp Med 123(2):239–250
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI (2012) Human gut microbiome viewed across age and geography. Nature 486(7402):222–227
Ottman N, Smidt H, de Vos WM, Belzer C (2012) The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol 2:104. https://doi.org/10.3389/fcimb.2012.00104
Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW (2011) Colonic contribution to uremic solutes. J Am Soc Nephrol 22(9):1769–1776
Mair RD, Sirich TL, Plummer NS, Meyer TW (2018) Characteristics of colon-derived uremic solutes. Clin J Am Soc Nephrol 13(9):1398–1404
Vanholder R, Glorieux G (2018) Gut-derived metabolites and chronic kidney disease. The forest (f) or the trees? Clin J Am Soc Nephrol 13:1311–1313
Cosola C, Rocchetti MT, Cupisti A, Gesualdo L (2018) Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol Res 130:132–142
Li DY, Tang WHW (2018) Contributory role of gut microbiota and their metabolites toward cardiovascular complications in chronic kidney disease. Semin Nephrol 38(2):193–205
Lau WL, Savoj J, Nakata MB, Vaziri ND (2018) Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond) 132(5):509–522
Noel S, Martina-Lingua MN, Bandapalle S, Pluznick J, Hamad AR, Peterson DA, Rabb H (2014) Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract 127(1–4):139–143
Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Gonzalez-Parra E, Sanz AB, Ortiz A, Sanchez-Niño MD (2017) Nutrients turned into toxins: microbiota modulation of nutrient properties in chronic kidney disease. Nutrients 9(5):489
Kikuchi M, Ueno M, Itoh Y, Suda W, Hattori M (2017) Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 135(1):51–60
Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, Fouque D (2014) Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol 9(3):399–410
Chaves LD, McSkimming DI, Bryniarski MA, Honan AM, Abyad S, Thomas SA, Wells S, Buck M, Sun Y, Genco RJ, Quigg RJ, Yacoub R (2018) Chronic kidney disease, uremic milieu, and its effects on gut bacterial microbiota dysbiosis. Am J Physiol Renal Physiol 315(3):F487–F502
Koppe L, Fouque D, Soulage CO (2018) The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins (Basel) 10(4):155
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS (2016) Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis 67(3):483–498
Jankowski J, Westhof T, Vaziri ND, Ingrosso D, Perna AF (2014) Gases as uremic toxins: is there something in the air? Semin Nephrol 34(2):135–150
Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, Pollard KS, Sonnenburg JL, Fischbach MA (2016) Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe 20(6):709–715
Yacoub R, Wyatt CM (2017) Manipulating the gut microbiome to decrease uremic toxins. Kidney Int 91(3):521–523
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH (2016) Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol 310(9):F857–F871
Cupisti A, Brunori G, Di Iorio BR, D’Alessandro C, Pasticci F, Cosola C, Bellizzi V, Bolasco P, Capitanini A, Fantuzzi AL, Gennari A, Piccoli GB, Quintaliani G, Salomone M, Sandrini M, Santoro D, Babini P, Fiaccadori E, Gambaro G, Garibotto G, Gregorini M, Mandreoli M, Minutolo R, C (2018) Nutritional treatment of advanced CKD: twenty consensus statements. J Nephrol 31(4):457–473
Bellizzi V, Conte G, Borrelli S, Cupisti A, De Nicola L, Di Iorio BR, Cabiddu G, Mandreoli M, Paoletti E, Piccoli GB, Quintaliani G, Ravera M, Santoro D, Torraca S, Minutolo R, “Conservative Treatment of CKD” Study Group of the Italian Society of Nephrology (2017) Controversial issues in CKD clinical practice: position statement of the CKD-treatment working group of the Italian Society of Nephrology. J Nephrol 30(2):159–170
Di Iorio BR, Cupisti A, D’Alessandro C, Bellasi A, Barbera V, Di Lullo L (2018) Nutritional therapy in autosomal dominant polycystic kidney disease. J Nephrol 31(5):635–643
Cosola C, Rocchetti MT, Sabatino A, Fiaccadori E, Di Iorio BR, Gesualdo L (2018) Microbiota issue in CKD: how promising are gut-targeted approaches ? J Nephrol. https://doi.org/10.1007/s40620-018-0516-0
Rollino C, Vischini G, Coppo R (2016) IgA nephropathy and infections. J Nephrol 29(4):463–468
Black AP, Anjos JS, Cardozo L, Carmo FL, Dolenga CJ, Nakao LS, de Carvalho Ferreira D, Rosado A, Carraro Eduardo JC, Mafra D (2018) Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J Ren Nutr 28(3):208–214
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4(10):1551–1558
Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y (2006) Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69(6):1081–1087
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) (2010) Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transpl 25(4):1183–1191
Liabeuf S, Glorieux G, Lenglet A, Diouf M, Schepers E, Desjardins L, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin (EUTox) Work Group (2013) Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS One 8(6):e67168
Mishima E, Fukuda S, Mukawa C, Yuri A, Kanemitsu Y, Matsumoto Y, Akiyama Y, Fukuda NN, Tsukamoto H, Asaji K, Shima H, Kikuchi K, Suzuki C, Suzuki T, Tomioka Y, Soga T, Ito S, Abe T (2017) Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int 92(3):634–645
Vanholder R, Glorieux G (2015) The intestine and the kidneys: a bad marriage can be hazardous. Clin Kidney J 8(2):168–179
Streeter E, Ng HH, Hart JL (2013) Hydrogen sulfide as a vasculoprotective factor. Med Gas Res 3(1):9
Weber GJ, Pushpakumar S, Tyagi SC, Sen U (2016) Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res 113:300–312
Flannigan KL, McCoy KD, Wallace JL (2011) Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis. Am J Physiol Gastrointest Liver Physiol 301(1):G188–G193
Barton LL, Ritz NL, Fauque GD, Lin HC (2017) Sulfur cycling and the intestinal microbiome. Dig Dis Sci 62(9):2241–2257
Perna AF, Luciano MG, Ingrosso D, Pulzella P, Sepe I, Lanza D, Violetti E, Capasso R, Lombardi C, De Santo NG (2009) Hydrogen sulphide-generating pathways in haemodialysis patients: a study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol Dial Transpl 24(12):3756–3763
Aminzadeh MA, Vaziri ND (2012) Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transpl 27(2):498–504
Kuang Q, Xue N, Chen J, Shen Z, Cui X, Fang Y, Ding X (2018) Low plasma hydrogen sulfide is associated with impaired renal function and cardiac dysfunction. Am J Nephrol 47(5):361–371
Zacchia M, Capasso G (2015) The importance of uromodulin as regulator of salt reabsorption along the thick ascending limb. Nephrol Dial Transpl 30(2):158–160
Perna AF, Ingrosso D (2012) Low hydrogen sulphide and chronic kidney disease: a dangerous liaison. Nephrol Dial Transpl 27(2):486–493
Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO (2013) Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60:195–200
Perna AF, Di Nunzio A, Amoresano A, Pane F, Fontanarosa C, Pucci P, Vigorito C, Cirillo G, Zacchia M, Trepiccione F, Ingrosso D (2016) Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 126:97–107
Patrick J. Knerr, Wilfred A, van der Donk (2012) Discovery, biosynthesis, and engineering of lantipeptides. Annu Rev Biochem 81:479–505
Perna AF, Zacchia M, Trepiccione F, Ingrosso D (2017) The sulfur metabolite lanthionine: evidence for a role as a novel uremic toxin. Toxins (Basel) 9(1):26
Perna AF, Anishchenko E, Vigorito C, Zacchia M, Trepiccione F, D’Aniello S, Ingrosso D (2018) Zebrafish, a novel model system to study uremic toxins: the case for the sulfur amino acid lanthionine. Int J Mol Sci 19(5):E1323. https://doi.org/10.3390/ijms19051323
Perna AF, Ingrosso D, Satta E, Lombardi C, Acanfora F, De Santo NG (2004) Homocysteine metabolism in renal failure. Curr Opin Clin Nutr Metab Care 7(1):53–57
Perna AF, Ingrosso D, Satta E, Romano M, Cimmino A, Galletti P, Zappia V, De Santo NG (2001) Metabolic consequences of hyperhomocysteinemia in uremia. Am J Kidney Dis 38(4 Suppl 1):S85–S90
Perna AF, Acanfora F, Luciano MG, Pulzella P, Capasso R, Satta E, Cinzia L, Pollastro RM, Iannelli S, Ingrosso D, De Santo NG (2007) Plasma protein homocysteinylation in uremia. Clin Chem Lab Med 45(12):1678–1682
Capasso R, Sambri I, Cimmino A, Salemme S, Lombardi C, Acanfora F, Satta E, Puppione DL, Perna AF, Ingrosso D (2012) Homocysteinylated albumin promotes increased monocyte-endothelial cell adhesion and up-regulation of MCP1, Hsp60 and ADAM17. PLoS One 7(2):e31388
Perna AF, Ingrosso D, Satta E, Lombardi C, Galletti P, D’Aniello A, De Santo NG (2004) Plasma protein aspartyl damage is increased in hemodialysis patients: studies on causes and consequences. J Am Soc Nephrol 15(10):2747–2754
Perna AF, Castaldo P, De Santo NG, di Carlo E, Cimmino A, Galletti P, Zappia V, Ingrosso D (2001) Plasma proteins containing damaged L-isoaspartyl residues are increased in uremia: implications for mechanism. Kidney Int 59(6):2299–2308
Perna AF, D’Aniello A, Lowenson JD, Clarke S, De Santo NG, Ingrosso D (1997) D-aspartate content of erythrocyte membrane proteins is decreased in uremia: implications for the repair of damaged proteins. J Am Soc Nephrol 8(1):95–104
Hill MJ (1997) Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev 6(Suppl 1):S43–S45
Rossi M, Amaretti A, Raimondi S (2011) Folate production by probiotic bacteria. Nutrients 3(1):118–134
Gerhauser C (2018) Impact of dietary gut microbial metabolites on the epigenome. Philos Trans R Soc B 373(1748):20170359
Ingrosso D, Cimmino A, Perna AF, Masella L, De Santo NG, De Bonis ML, Vacca M, D’Esposito M, D’Urso M, Galletti P, Zappia V (2003) Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 361(9370):1693–1699
Perna AF, Lanza D, Sepe I, Conzo G, Altucci L, Ingrosso D (2013) Altered folate receptor 2 expression in uraemic patients on haemodialysis: implications for folate resistance. Nephrol Dial Transpl 28:1214–1224
Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, Wang B, Huo Y, Hou FF, investigators of the Renal Substudy of the China Stroke Primary Prevention Trial (CSPPT) (2016) Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the china stroke primary prevention trial. JAMA Intern Med 176(10):1443–1450
Wyatt CM, Spence JD (2016) Folic acid supplemetation and chronic kidney disease progression. Kidney Int 90:1142–1145
Feng SJ, Li H, Wang SX (2015) Lower hydrogen sulfide is associated with cardiovascular mortality, which involves cPKCβII/Akt pathway in chronic hemodialysis patients. Blood Purif 240(3):260–269. https://doi.org/10.1159/000439580
Wu D, Luo N, Wang L, Zhao Z, Bu H, Xu G, Yan Y, Che X, Jiao Z, Zhao T, Chen J, Ji A, Li Y, Lee GD (2017) Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci Rep 7(1):455
Zhang L, Wang Y, Li Y, Li L, Xu S, Feng X, Liu S (2018) Hydrogen sulfide (H2S)-releasing compounds: therapeutic potential in cardiovascular diseases. Front Pharmacol 9:1066
Giustarini D, Tazzari V, Bassanini I, Rossi R, Sparatore A (2018) The new H2S-releasing compound ACS94 exerts protective effects through the modulation of thiol homoeostasis. J Enzyme Inhib Med Chem 33(1):1392–1404
Hsu C, Tain Y (2019) Hydrogen sulfide in hypertension and kidney disease of developmental origins. J Mol Sci 19:1438. https://doi.org/10.3390/ijms19051438
Vicente JB, Malagrinò F, Arese M, Forte E, Sarti P, Giuffrè A (2016) Bioenergetic relevance of hydrogen sulfide and the interplay between gasotransmitters at human cystathionine β-synthase. Biochim Biophys Acta 1857(8):1127–1138
Acknowledgements
We thank the European Uremic Toxin (EUTox) Work Group for supporting our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, except for AFP, who received research fundings from Gnosis S.p.A and EUTox.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perna, A.F., Glorieux, G., Zacchia, M. et al. The role of the intestinal microbiota in uremic solute accumulation: a focus on sulfur compounds. J Nephrol 32, 733–740 (2019). https://doi.org/10.1007/s40620-019-00589-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00589-z