Abstract
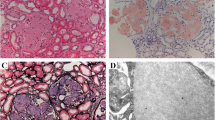
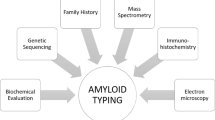
Renal amyloidosis is characterized by acellular Congo red positive deposits in the glomeruli, interstitium and/or arteries. Light chain restriction on immunofluorescence studies is present in AL-amyloidosis, the most common type of amyloidosis involving the kidney. The detection of Congo red positive deposits coupled with negative immunofluorescence studies is highly suggestive of non-AL amyloidosis. Some of the non-AL amyloidosis are common while others are relatively rare. The clinical features, laboratory and renal pathology findings are helpful in the diagnosis and typing of non-AL amyloidosis. Thus, ALECT2 amyloidosis is characterized by diffuse cortical interstitial amyloid deposits, AA amyloidosis shows vascular deposits in addition to the glomerular deposits, AFib amyloidosis is characterized by massive amyloid accumulation limited to the glomeruli resulting in the obliteration of glomerular architecture, AApoA1 and AApoAIV are characterized by large amyloid deposits restricted to the medulla, and AGel shows swirling patterns of amyloid fibrils on electron microscopy. While light microscopy is very helpful, accurate typing of non-AL amyloidosis then requires immunohistochemical or laser microdissection/mass spectrometry studies of the Congo red positive deposits. Immunohistochemical studies are available for some of the non-AL amyloidosis. On the other hand, mass spectrometry analysis is a one stop methodology for confirmation and typing of amyloidosis. The diagnosis and typing of amyloidosis by mass spectrometry is based on finding the signature amyloid peptides, apolipoprotein E and serum amyloid-P component, followed by detection of precursor amyloidogenic protein such as LECT2, fibrinogen-α, gelsolin, etc. To, summarize, non-AL amyloidosis is a group of amyloidosis with distinctive clinical, laboratory and renal pathology findings. Typing of the amyloidosis is best performed using mass spectrometry methodology. Accurate typing of non-AL amyloidosis is imperative for correct management, prognosis, and genetic counseling.


Similar content being viewed by others
References
Dember LM (2006) Amyloidosis-associated kidney disease. J Am Soc Nephrol 17:3458–3471
Merlini G, Bellotti V (2003) Molecular mechanisms of amyloidosis. N Engl J Med 349:583–596
Said SM, Sethi S, Valeri AM et al (2013) Renal amyloidosis: origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol 8:1515–1523
Sethi S, Theis JD, Leung N et al (2010) Mass spectrometry based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol 5:2180–2187
Picken MM (2007) New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens 16:196–203
Vigushin DM, Gough J, Allan D et al (1994) Familial nephropathic systemic amyloidosis caused by apolipoprotein Al variant Arg26. QJM 87:149–154
Yazaki M, Liepnieks JJ, Barats MS, Cohen AH, Benson MD (2003) Hereditary systemic amyloidosis associated with a new apolipoprotein AII stop codon mutation Stop78Arg. Kidney Int 64:11–16
Yazaki M, Liepnieks JJ, Yamashita T, Guenther B, Skinner M, Benson MD (2001) Renal amyloidosis caused by a novel stop-codon mutation in the apolipoprotein A-II gene. Kidney Int 60:1658–1665
Benson M JL, Uemichi T, Wheeler G, Correa R (1993) Hereditary renal amyloidosis associated with a mutant fibrinogen [alpha]-chain. Blood 3:252–255
Benson MD (2005) Ostertag revisited: the inherited systemic amyloidoses without neuropathy. Amyloid 12:75–87
Joy T, Wang J, Hahn A, Hegele RA (2003) Apoa1 related amyloidosis: a case report and literature review. Clin Biochem 36:641–645
Gillmore JD, Booth DR, Madhoo S, Pepys MB, Hawkins PN (1999) Hereditary renal amyloidosis associated with variant lysozyme in a large English family. Nephrol Dial Transplant 14:2639–2644
Dasari S, Amin MS, Kurtin PJ et al. Clinical, biopsy, and mass spectrometry characteristics of renal apolipoprotein A-IV amyloidosis. Kidney International;90:658–664
Sethi S, Dasari S, Amin MS et al (2017) Clinical, biopsy, and mass spectrometry findings of renal gelsolin amyloidosis. Kidney Int 91:964–971
Lachmann HJ, Goodman HJB, Gilbertson JA et al (2007) Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356:2361–2371
Chugh KS, Datta, Singhal PC, Jain S, Sakhuja V, Dash SC (1981) Pattern of renal amyloidosis in indian patients. Postgrad Med J 57:31–35
Verine J, Mourad N, Desseaux K et al (2007) Clinical and histological characteristics of renal AA amyloidosis: a retrospective study of 68 cases with a special interest to amyloid-associated inflammatory response. Hum Pathol 38:1798–1809
Saha A, Chopra Y, Theis JD, Vrana JA, Sethi S (2013) AA amyloidosis associated with systemic-onset juvenile idiopathic arthritis. Am J Kidney Dis 62:834–838
Lane T, Loeffler JM, Rowczenio DM et al (2013) Brief report: AA amyloidosis complicating the hereditary periodic fever syndromes. Arthritis Rheum 65:1116–1121
Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B (2008) Leukocyte chemotactic factor 2: A novel renal amyloid protein. Kidney Int 74:218–222
Said SM, Sethi S, Valeri AM et al (2014) Characterization and outcomes of renal leukocyte chemotactic factor 2-associated amyloidosis. Kidney Int 86:370–377
Nasr SH, Dogan A, Larsen CP (2015) Leukocyte cell–derived chemotaxin 2–associated amyloidosis: a recently recognized disease with distinct clinicopathologic characteristics. Clin J Am Soc Nephrol 10:2084–2093
Paueksakon P, Fogo AB, Sethi S (2014) Leukocyte chemotactic factor 2 amyloidosis cannot be reliably diagnosed by immunohistochemical staining. Hum Pathol 45:1445–1450
Gillmore JD, Lachmann HJ, Rowczenio D et al (2009) Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen Aα-chain amyloidosis. J Am Soc Nephrol 20:444–451
Uemichi T, Liepnieks JJ, Benson MD (1994) Hereditary renal amyloidosis with a novel variant fibrinogen. J Clin Invest 93:731–736
Stangou AJ, Banner NR, Hendry BM et al (2010) Hereditary fibrinogen A α-chain amyloidosis: phenotypic characterization of a systemic disease and the role of liver transplantation. Blood 115:2998–3007
Gillmore JD, Booth DR, Rela M et al (2000) Curative hepatorenal transplantation in systemic amyloidosis caused by the Glu526Val fibrinogen {alpha}-chain variant in an English family. QJM 93:269–275
Sethi S, Ters ME, Vootukuru S, Qian Q (2011) Recurrent AA amyloidosis in a kidney transplant. Am J Kidney Dis 57:941–944
Meretoja J (1969) Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms. A previously unrecognized heritable syndrome. Ann Clin Res 1:314–324
Maury CPJ, Alii K, Baumann M (1990) Finnish hereditary amyloidosis. FEBS Lett 260:85–87
Maury CPJ, Kere J, Tolvanen R, de la Chapelle A (1990) Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene. FEBS Lett 276:75–77
Maury CPJ, Kere J, Tolvanen R, de la Chapelle A (1992) Homozygosity for the Asn187 gelsolin mutation in Finnish-type familial amyloidosis is associated with severe renal disease. Genomics 13:902–903
Sethi S, Theis JD, Quint P et al (2013) Renal Amyloidosis Associated With a Novel Sequence Variant of Gelsolin. Am J Kidney Dis 61:161–166
Gregorini G, Izzi C, Ravani P et al (2015) Tubulointerstitial nephritis is a dominant feature of hereditary apolipoprotein A-I amyloidosis. Kidney Int 87:1223–1229
Rowczenio D, Dogan A, Theis JD et al (2011) Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am J Pathol 179:1978–1987
Gregorini G, Izzi C, Obici L et al (2005) Renal apolipoprotein a-i amyloidosis: a rare and usually ignored cause of hereditary tubulointerstitial nephritis. J Am Soc Nephrol 16:3680–3686
Obici L, Palladini G, Giorgetti S et al (2004) Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated Italian families. Gastroenterology 126:1416–1422
Traynor CA, Tighe D, O’Brien FJ et al (2013) Clinical and pathologic characteristics of hereditary apolipoprotein A-I amyloidosis in Ireland. Nephrology 18:549–554
Gillmore JD, Stangou AJ, Lachmann HJ et al (2006) Organ Transplantation in Hereditary Apolipoprotein AI Amyloidosis. Am J Transplant 6:2342–2347
De Gracia R, Fernández EJ, Riñón C, Selgas R, Garcia-Bustos J (2006) Hereditary renal amyloidosis associated with a novel mutation in the apolipoprotein AII gene. QJM: An International. J Med 99:274-
Benson MD, Liepnieks JJ, Yazaki M et al (2001) A new human hereditary amyloidosis: the result of a stop-codon mutation in the apolipoprotein AII gene. Genomics 72:272–277
Sethi S, Theis JD, Shiller SM et al (2012) Medullary amyloidosis associated with apolipoprotein A-IV deposition. Kidney Int 81:201–206
Nasr SH, Dasari S, Hasadsri L et al (2017) Novel type of renal amyloidosis derived from apolipoprotein-CII. J Am Soc Nephrol 28:439–445
Valleix S, Drunat S, Philit J-B et al (2002) Hereditary renal amyloidosis caused by a new variant lysozyme W64R in a French family. Kidney Int 61:907–912
Granel B, Valleix S, Serratrice J et al (2006) Lysozyme amyloidosis: report of 4 cases and a review of the literature. Medicine (Baltimore) 85:66–73
Nasr SH, Dasari S, Mills JR et al (2017) Hereditary lysozyme amyloidosis variant p.Leu102Ser associates with unique phenotype. J Am Soc Nephrol 28:431–438
Sethi S, Vrana JA, Theis JD, Dogan A (2013) Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 22:273–280
Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, Dogan A (2009) Classification of amyloidosis by laser microdissection and mass spectrometry based proteomic analysis in clinical biopsy specimens. Blood 114:4957–4959
Dasari S, Theis JD, Vrana JA et al (2014) Clinical proteome informatics workbench detects pathogenic mutations in hereditary amyloidoses. J Proteome Res 13:2352–2358
Sethi S, Vrana JA, Theis JD et al (2012) Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 82:226–234
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sethi, S., Theis, J.D. Pathology and diagnosis of renal non-AL amyloidosis. J Nephrol 31, 343–350 (2018). https://doi.org/10.1007/s40620-017-0426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-017-0426-6