Abstract
Aim
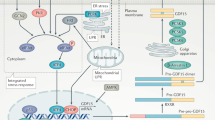
Growth differentiation factor 15 (GDF15) is a stress response cytokine that has been proposed as a relevant metabolic hormone. Descriptive studies have shown that plasma GDF15 levels are regulated by short term changes in nutritional status, such as fasting, or in obesity. However, few data exist regarding how GDF15 levels are regulated in peripheral tissues. The aim of the present work was to study the variations on gastric levels of GDF15 and its precursor under different physiological conditions, such as short-term changes in nutritional status or overfeeding achieved by HFD. Moreover, we also address the sex- and age-dependent alterations in GDF15 physiology.
Methods
The levels of gastric and plasma GDF15 and its precursor were measured in lean and obese mice, rats and humans by western blot, RT-PCR, ELISA, immunohistochemistry and by an in vitro organ culture system.
Results
Our results show a robust regulation of gastric GDF15 production by fasting in rodents. In obesity an increase in GDF15 secretion from the stomach is reflected with an increase in circulating levels of GDF15 in rats and humans. Moreover, gastric GDF15 levels increase with age in both rats and humans. Finally, gastric GDF15 levels display sexual dimorphism, which could explain the difference in circulating GFD15 levels between males and females, observed in both humans and rodents.
Conclusions
Our results provide clear evidence that gastric GDF15 is a critical contributor of circulating GDF15 levels and can explain some of the metabolic effects induced by GDF15.






Similar content being viewed by others
References
Williams EP et al (2015) Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 4(3):363–370
Folgueira C, Seoane LM, Casanueva FF (2014) The brain-stomach connection. Front Horm Res 42:83–92
Evers SS, Sandoval DA, Seeley RJ (2017) The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev Physiol 79:313–334
Quinones M et al (2019) Exciting advances in GPCR-based drugs discovery for treating metabolic disease and future perspectives. Expert Opin Drug Discov 14(5):421–431
Muller TD, Tschop MH (2022) Gut-hormone triple agonists: clinical safety and metabolic benefits. Lancet 400(10366):1826–1828
Bootcov MR et al (1997) MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 94(21):11514–11519
Xiong Y et al (2017) Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aan8732
Patel S et al (2019) GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab 29(3):707-718e8
Fagerberg L et al (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom 13(2):397–406
GTEx Portal (n.d.) https://gtexportal.org/home/gene/GDF15
Macia L et al (2012) Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS ONE 7(4):e34868
Tsai VW et al (2013) TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS ONE 8(2):e55174
Sjoberg KA et al (2023) GDF15 increases insulin action in the liver and adipose tissue via a beta-adrenergic receptor-mediated mechanism. Cell Metab 35(8):1327-1340e5
Asrih M et al (2023) Overview of growth differentiation factor 15 in metabolic syndrome. J Cell Mol Med 27(9):1157–1167
Wang D et al (2023) GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 619(7968):143–150
Breit SN et al (2023) GDF15 enhances body weight and adiposity reduction in obese mice by leveraging the leptin pathway. Cell Metab 35(8):1341-1355e3
Bauskin AR et al (2000) The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J 19(10):2212–2220
Bauskin AR et al (2010) The TGF-beta superfamily cytokine MIC-1/GDF15: secretory mechanisms facilitate creation of latent stromal stores. J Interferon Cytokine Res 30(6):389–397
Baek SJ, Eling T (2019) Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther 198:46–58
Emmerson PJ et al (2017) The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23(10):1215–1219
Hsu JY et al (2017) Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550(7675):255–259
Mullican SE et al (2017) GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23(10):1150–1157
Yang L et al (2017) GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23(10):1158–1166
Kalia M, Mesulam MM (1980) Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193(2):467–508
Kalia M, Welles RV (1980) Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res 188(1):23–32
Al-Massadi O et al (2010) Age, sex, and lactating status regulate ghrelin secretion and GOAT mRNA levels from isolated rat stomach. Am J Physiol Endocrinol Metab 299(3):E341–E350
Senin LL et al (2015) Regulation of NUCB2/nesfatin-1 production in rat’s stomach and adipose tissue is dependent on age, testosterone levels and lactating status. Mol Cell Endocrinol 411:105–112
Folgueira C et al (2017) Pharmacological inhibition of cannabinoid receptor 1 stimulates gastric release of nesfatin-1 via the mTOR pathway. World J Gastroenterol 23(35):6403–6411
Seoane LM et al (2007) Sensory stimuli directly acting at the central nervous system regulate gastric ghrelin secretion. An ex vivo organ culture study. Endocrinology 148(8):3998–4006
Seoane LM et al (2007) Growth hormone and somatostatin directly inhibit gastric ghrelin secretion. An in vitro organ culture system. J Endocrinol Investig 30(9):RC22–RC25
Senin LL et al (2013) The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS ONE 8(11):e80339
Al Massadi O et al (2010) Macronutrients act directly on the stomach to regulate gastric ghrelin release. J Endocrinol Invest 33(9):599–602
Salman A et al (2021) Changes in plasma growth differentiation factor-15 after laparoscopic sleeve gastrectomy in morbidly obese patients: a prospective study. J Inflamm Res 14:1365–1373
Tanaka T et al (2018) Plasma proteomic signature of age in healthy humans. Aging Cell 17(5):e12799
Conte M et al (2019) Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci 74(5):600–607
Lehallier B et al (2019) Undulating changes in human plasma proteome profiles across the lifespan. Nat Med 25(12):1843–1850
Kim H et al (2020) Growth differentiation factor-15 as a biomarker for sarcopenia in aging humans and mice. Exp Gerontol 142:111115
Tavenier J et al (2021) Longitudinal course of GDF15 levels before acute hospitalization and death in the general population. Geroscience 43(4):1835–1849
Kasacka I et al (2012) Immunohistochemical identification and localisation of gastrin and somatostatin in endocrine cells of human pyloric gastric mucosa. Folia Morphol (Warsz) 71(1):39–44
Cammisotto P, Bendayan M (2012) A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol 45(1):1–16
Klein AB et al (2021) Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat Commun 12(1):1041
Schernthaner-Reiter MH et al (2016) Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity. Eur J Endocrinol 175(6):623–631
Stengel A et al (2009) Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150(1):232–238
Shi Y et al (2023) A rat model of metabolic syndrome-related heart failure with preserved ejection fraction phenotype: pathological alterations and possible molecular mechanisms. Front Cardiovasc Med 10:1208370
Alcazar J et al (2021) Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: the Copenhagen Sarcopenia Study. J Cachexia Sarcopenia Muscle 12(6):1418–1427
Welsh P et al (2022) Reference ranges for GDF-15, and risk factors associated with GDF-15, in a large general population cohort. Clin Chem Lab Med 60(11):1820–1829
Barja-Fernandez S et al (2015) Peripheral signals mediate the beneficial effects of gastric surgery in obesity. Gastroenterol Res Pract 2015:560938
Funding
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the grant number PI22/00202 (L.M.S.) and PI21/01216 (OA-M) and co-funded by the European Union. GAIN-XUNTA de Galicia, Proyectos de Excelencia [IN607D 2023/02 (M.Q.), IN607D-2022-07 (O.A.-M.) and IN607D-2022-04 (L.M.S.)]; Ministerio de Ciencia e Innovación and “FEDER” PID2022-142084OA-100 (M.Q.); Fundación de la Sociedad Gallega de Endocrinología y Nutrición (OA-M); Centro de Investigación Biomédica en Red (CIBER) de Fisiopatología de la Obesidad y Nutrición (CIBERobn). CIBERobn is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain, which is supported by FEDER funds; E.P. and RP-L hold a IDIS fellowship. OA-M and MQ were funded by a research contract Miguel Servet (CP20/00146 CP21/00108 respectively) from the ISCIII. The data figures were generated by using BioRender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization, OA-M, LMS, formal Analysis, VP-L, RP-L, investigation, VP-L, RP-L, MV, CF, SB-F, EP, TG-C, JB, FS, MQ; writing–original draft, VP-L, RN, OA-M, and LMS; writing–review and editing, VP-L, JF, RN, MQ, OA-M and LMS; funding acquisition, LMS, OA.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The authors declare they have no financial interests.
Research involving human participants and/or animals
This study was conducted in accordance with the guidelines in the Declaration of Helsinki and approved by the Ethics and Research Committee of the Autonomous Community of Galicia Community under code 2013/256.
Informed consent
All samples were obtained with the informed consent of all included patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pena-Leon, V., Perez-Lois, R., Villalon, M. et al. Gastric GDF15 levels are regulated by age, sex, and nutritional status in rodents and humans. J Endocrinol Invest 47, 1139–1154 (2024). https://doi.org/10.1007/s40618-023-02232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02232-y