Abstract
Purpose
Prior research has focused on glucose/insulin responses to meal challenges to create personalized diets to improve health, though it is unclear if these responses predict chronic diseases. We aimed to identify glucose and insulin responses to a mixed meal tolerance test (MMTT) that predict the development of diabetic retinopathy (DR) and compare the predictive abilities with the oral glucose tolerance test (OGTT).
Methods
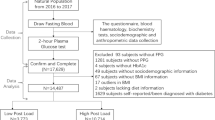
Indigenous American adults without diabetes (n = 168) underwent a 4-h MMTT, body composition assessment, and a 3-h OGTT at baseline. During follow-up (median 13.4 years), DR was diagnosed by direct ophthalmoscopy (n = 28) after onset of type 2 diabetes. Total and incremental area under the curve (AUC and iAUC) were calculated from glucose/insulin responses after the MMTT and OGTT.
Results
In separate Cox proportional hazards models adjusted for age, sex, and body fat (%), MMTT glucose AUCs (180-min and 240-min) and iAUC (180-min) predicted DR (HR 1.50, 95% CI 1.06, 2.12; HR 1.50, 95% CI 1.05, 2.14; HR 1.58, 95% CI 1.01, 2.46). The predictive abilities were better than the fasting OGTT glucose (p < 0.01) but similar to the 120-min OGTT glucose (p = 0.53). MMTT insulin AUCs (180-min and 240-min) and iAUC (180-min) also predicted DR (HR 1.65, 95% CI 1.09, 2.51; HR 1.58, 95% CI 1.00, 2.35; HR 1.53 95% CI 1.06, 2.22) while insulin AUC and iAUC from the OGTT did not (p > 0.05).
Conclusions
Higher MMTT glucose and insulin responses predicted DR and were comparable to the OGTT, supporting the use of a meal challenge for precision nutrition.
Trial registrations: Clinical Trial Registry: ClinicalTrials.gov identifier: NCT00340132, NCT00339482.


Similar content being viewed by others
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations
- AUC:
-
Area under the curve
- DR:
-
Diabetic retinopathy
- DXA:
-
Dual-energy X-ray absorptiometry
- iAUC:
-
Incremental area under the curve
- MMTT:
-
Mixed meal tolerance test
- OGTT:
-
Oral glucose tolerance test
References
Wopereis S et al (2017) Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: the PhenFlex challenge. Genes Nutr 12:21. https://doi.org/10.1186/s12263-017-0570-6
Stroeve JHM, van Wietmarschen H, Kremer BHA, van Ommen B, Wopereis S (2015) Phenotypic flexibility as a measure of health: the optimal nutritional stress response test. Genes Nutr 10(3):13. https://doi.org/10.1007/s12263-015-0459-1
van Ommen B, van der Greef J, Ordovas JM, Daniel H (2014) Phenotypic flexibility as key factor in the human nutrition and health relationship. Genes Nutr 9(5):423. https://doi.org/10.1007/s12263-014-0423-5
Jagannathan R et al (2020) The oral glucose tolerance test: 100 years later. Diabetes Metab Syndr Obes 13:3787–3805. https://doi.org/10.2147/DMSO.S246062
Dorf A, Ballintine EJ, Bennett PH, Miller M (1976) Retinopathy in Pima Indians. Relationships to glucose level, duration of diabetes, age at diagnosis of diabetes, and age at examination in a population with a high prevalence of diabetes mellitus. Diabetes 25(7):554–560. https://doi.org/10.2337/diab.25.7.554
Rushforth NB, Miller M, Bennett PH (1979) Fasting and two-hour post-load glucose levels for the diagnosis of diabetes. Diabetologia 16(6):373–379. https://doi.org/10.1007/bf01223157
Gabir MM et al (2000) Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care 23(8):1113–1118. https://doi.org/10.2337/diacare.23.8.1113
Xin Z et al (2012) Evaluation for fasting and 2-hour glucose and HbA1C for diagnosing diabetes based on prevalence of retinopathy in a Chinese population. PLoS ONE 7(7):e40610. https://doi.org/10.1371/journal.pone.0040610
Rushforth NB, Bennett PH, Steinberg AG, Miller M (1975) Comparison of the value of the two- and one-hour glucose levels of the oral GTT in diagnosis of diabetes in Pima Indians. Diabetes 24(6):538–546
Mitchell CM SE, Chang DC, Krakoff J. Utility of a mixed meal test in predicting type 2 diabetes risk. Presented at the The Obesity Society, San Diego; 2022.
Stinson EJ PP, Mitchell CM, Krakoff J. Reproducibility and determinants of the metabolic responses during a mixed meal tolerance test. Presented at the The Obesity Society, San Diego, CA, 2022.
Berry SE et al (2020) Human postprandial responses to food and potential for precision nutrition. Nat Med 26(6):964–973. https://doi.org/10.1038/s41591-020-0934-0
Department of Health and Human Services. Nutrition for precision health. https://grants.nih.gov/grants/guide/rfa-files/RFA-RM-21-005.html. Accessed 29 July 2022.
Zeevi D et al (2015) Personalized nutrition by prediction of glycemic responses. Cell 163(5):1079–1094. https://doi.org/10.1016/j.cell.2015.11.001
Roche HM (2021) Postprandial metabolism and inflammation-a comprehensive model to advance Precision Nutrition? Lessons learned from the Personalised REsponses to DIetary Composition Trial (PREDICT study). Am J Clin Nutr 114(3):841–842. https://doi.org/10.1093/ajcn/nqab200
Lee BY et al (2022) Research gaps and opportunities in precision nutrition: an NIH workshop report. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqac237
Lillioja S et al (1993) Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329(27):1988–1992. https://doi.org/10.1056/NEJM199312303292703
Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E (1991) Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 53(6):1368–1371. https://doi.org/10.1093/ajcn/53.6.1368
Genuth S et al (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26(11):3160–3167. https://doi.org/10.2337/diacare.26.11.3160
Alferes VR, Alferes VR (2012) Methods of randomization in experimental design. SAGE, Los Angeles (in English)
Herbert V, Lau K, Gottlieb CW, Bleicher SJ (1965) Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25(10):1374–1384
Yalow RS, Berson A (1996) Immunoassay of endogenous plasma insulin in man. Obes Res 4(6):583–600
Tataranni PA, Ravussin E (1995) Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 62(4):730–734. https://doi.org/10.1093/ajcn/62.4.730
Guo Y, Franks PW, Brookshire T, Antonio Tataranni P (2004) The intra- and inter-instrument reliability of DXA based on ex vivo soft tissue measurements. Obes Res 12(12):1925–1929. https://doi.org/10.1038/oby.2004.241
Goldman RFBE. Body Volume measurement by underwater weighing: description of a method. Washington DC; 1961.
Paddock E, Looker HC, Piaggi P, Knowler WC, Krakoff J, Chang DC (2018) One-hour plasma glucose compared with two-hour plasma glucose in relation to diabetic retinopathy in American Indians. Diabetes Care 41(6):1212–1217. https://doi.org/10.2337/dc17-1900
Knowler WC, Bennett PH, Ballintine EJ (1980) Increased incidence of retinopathy in diabetics with elevated blood pressure. A six-year follow-up study in Pima Indians. N Engl J Med 302(12):645–650. https://doi.org/10.1056/NEJM198003203021201
Pencina MJ, D’Agostino RB (2015) Evaluating discrimination of risk prediction models: the c statistic. JAMA 314(10):1063–1064. https://doi.org/10.1001/jama.2015.11082
Pencina MJ, D’Agostino RB (2004) Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 23(13):2109–2123. https://doi.org/10.1002/sim.1802
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the are under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845. https://doi.org/10.2307/2531595
Berry SE et al (2020) Publisher Correction: Human postprandial responses to food and potential for precision nutrition. Nat Med 26(11):1802. https://doi.org/10.1038/s41591-020-1130-y
Merino J (2022) Precision nutrition in diabetes: when population-based dietary advice gets personal. Diabetologia 65(11):1839–1848. https://doi.org/10.1007/s00125-022-05721-6
Voruganti VS (2023) Precision nutrition: recent advances in obesity. Physiology (Bethesda). https://doi.org/10.1152/physiol.00014.2022
Achour L, Meance S, Briend A (2001) Comparison of gastric emptying of a solid and a liquid nutritional rehabilitation food. Eur J Clin Nutr 55(9):769–772. https://doi.org/10.1038/sj.ejcn.1601221
Acknowledgements
We thank the participants and the research staff of the Phoenix Epidemiology and Clinical Research Branch.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. DK069015-36).
Author information
Authors and Affiliations
Contributions
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. E.J.S. and C.M.M. analyzed the data, contributed to discussion, and wrote, reviewed, and edited the manuscript. H.C.L., J.K, and D.C.C. contributed to discussion, and reviewed and edited the manuscript. All authors approved the final version of the manuscript. D.C.C. is the guarantor and therefore takes full responsibility for the work as a whole, including access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
On behalf of all authors, the corresponding author states that there is no conflict of interest. The study was previously approved by the NIDDK Institutional Review Board. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stinson, E.J., Mitchell, C.M., Looker, H.C. et al. Higher glucose and insulin responses to a mixed meal are associated with increased risk of diabetic retinopathy in Indigenous Americans. J Endocrinol Invest 47, 699–707 (2024). https://doi.org/10.1007/s40618-023-02187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02187-0