Abstract
Purpose
To evaluate the impact of pasireotide (PAS) therapy on hormonal and glycometabolic outcome in patients with acromegaly previously treated with combination medical therapies or unconventional dosages of first-generation somatostatin receptor ligands (fg-SRLs).
Methods
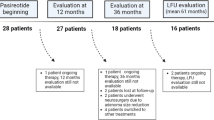
Retrospective study carried out in two referral centers for pituitary diseases. Twenty-one acromegalic patients were switched to PAS (12 had biochemical control, 9 were uncontrolled). Data were collected after 3- and 6-months PAS treatment, and at the last available visit (median 35 months).
Results
After switching to PAS therapy, a significant reduction in IGF-1 values was observed [median 39%; 0.79 xULN (IQR 0.5–1.01) vs 1.29 xULN (IQR 1.06–1.83); p = 0.009]. IGF-1 reduction was statistically significant in the 9 patients previously uncontrolled (61%, p = 0.016), and in the 12 controlled subjects (33%, p = 0.037). At last follow-up, the number of patients reaching an acceptable biochemical control (IGF-1 < 1.3 xULN) raised from 57 to 90% (p = 0.032). Mean HbA1c levels increased from 5.7% (5.5–5.9) to 6.0% (5.9–7) (p = 0.002), and the percentage of diabetic patients raised from 14% (3/21) to 67% (14/21) (p = 0.004). At the last evaluation HbA1c was ≥ 7.0% in 5 patients (24%). Antidiabetic drugs were initiated in 9 new patients, and in 7 out of 9 metformin alone was effective. Younger age and male sex were predictors for the maintenance of glucose homeostasis.
Conclusion
PAS monotherapy can be effective in acromegalic patients previously treated with combination medical therapies or unconventional dosages of fg-SRLs. Glucose imbalance can be managed in the vast majority of cases by use of lifestyle interventions and metformin.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available upon reasonable request to the corresponding author.
References
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202. https://doi.org/10.1172/JCI39375
Crisafulli S, Luxi N, Sultana J et al (2021) Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur J Endocrinol 185(2):251–263. https://doi.org/10.1530/EJE-21-0216
Melmed S (2006) Medical progress: Acromegaly. N Engl J Med 355(24):2558–2573. https://doi.org/10.1056/NEJMra062453
Holdaway IM, Bolland MJ, Gamble GD (2008) A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol 159(2):89–95. https://doi.org/10.1530/EJE-08-0267
Corica G, Ceraudo M, Campana C et al (2020) Octreotide-resistant acromegaly: challenges and solutions. Ther Clin Risk Manag 16:379–391. https://doi.org/10.2147/TCRM.S183360
Gatto F, Trifiro G, Lapi F et al (2018) Epidemiology of acromegaly in Italy: analysis from a large longitudinal primary care database. Endocrine 61(3):533–541. https://doi.org/10.1007/s12020-018-1630-4
Melmed S, Bronstein MD, Chanson P et al (2018) A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14(9):552–561. https://doi.org/10.1038/s41574-018-0058-5
Giustina A, Chanson P, Kleinberg D et al (2014) Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol 10(4):243–248. https://doi.org/10.1038/nrendo.2014.21
Gatto F, Campana C, Cocchiara F et al (2019) Current perspectives on the impact of clinical disease and biochemical control on comorbidities and quality of life in acromegaly. Rev Endocr Metab Disord 20(3):365–381. https://doi.org/10.1007/s11154-019-09506-y
Mazziotti G, Battista C, Maffezzoni F et al (2020) Treatment of acromegalic osteopathy in real-life clinical practice: the BAAC (Bone Active Drugs in Acromegaly) study. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa363
Babu H, Ortega A, Nuno M et al (2017) Long-term endocrine outcomes following endoscopic endonasal transsphenoidal surgery for acromegaly and associated prognostic factors. Neurosurgery 81(2):357–366. https://doi.org/10.1093/neuros/nyx020
Colao A, Auriemma RS, Pivonello R et al (2016) Interpreting biochemical control response rates with first-generation somatostatin analogues in acromegaly. Pituitary 19(3):235–247. https://doi.org/10.1007/s11102-015-0684-z
Colao A, Auriemma RS, Lombardi G et al (2011) Resistance to somatostatin analogs in acromegaly. Endocr Rev 32(2):247–271. https://doi.org/10.1210/er.2010-0002
Gadelha MR, Wildemberg LE, Kasuki L (2022) The future of somatostatin receptor ligands in acromegaly. J Clin Endocrinol Metab 107(2):297–308. https://doi.org/10.1210/clinem/dgab726
Gadelha MR, Wildemberg LE, Bronstein MD et al (2017) Somatostatin receptor ligands in the treatment of acromegaly. Pituitary 20(1):100–108. https://doi.org/10.1007/s11102-017-0791-0
Fleseriu M, Biller BMK, Freda PU et al (2021) A pituitary society update to acromegaly management guidelines. Pituitary 24(1):1–13. https://doi.org/10.1007/s11102-020-01091-7
Cocchiara F, Campana C, Nista F et al (2022) Evaluation of acromegaly treatment direct costs with respect to biochemical control and follow-up length. Pituitary 25(2):246–257. https://doi.org/10.1007/s11102-021-01193-w
Giustina A, Bonadonna S, Bugari G et al (2009) High-dose intramuscular octreotide in patients with acromegaly inadequately controlled on conventional somatostatin analogue therapy: a randomised controlled trial. Eur J Endocrinol 161(2):331–338. https://doi.org/10.1530/EJE-09-0372
Giustina A, Mazziotti G, Cannavo S et al (2017) High-dose and high-frequency Lanreotide Autogel in acromegaly: a randomized Multicenter Study. J Clin Endocrinol Metab 102(7):2454–2464. https://doi.org/10.1210/jc.2017-00142
Colao A, Pivonello R, Auriemma RS et al (2007) Beneficial effect of dose escalation of octreotide-LAR as first-line therapy in patients with acromegaly. Eur J Endocrinol 157(5):579–587. https://doi.org/10.1530/EJE-07-0383
Auriemma RS, Pivonello R, Ferreri L et al (2015) Cabergoline use for pituitary tumors and valvular disorders. Endocrinol Metab Clin North Am 44(1):89–97. https://doi.org/10.1016/j.ecl.2014.10.007
Sandret L, Maison P, Chanson P (2011) Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab 96(5):1327–1335. https://doi.org/10.1210/jc.2010-2443
Coopmans EC, van Meyel SWF, van der Lely AJ et al (2019) The position of combined medical treatment in acromegaly. Arch Endocrinol Metab 63(6):646–652. https://doi.org/10.20945/2359-3997000000195
Fleseriu M, Fuhrer-Sakel D, van der Lely AJ et al (2021) More than a decade of real-world experience of pegvisomant for acromegaly: ACROSTUDY. Eur J Endocrinol 185(4):525–538. https://doi.org/10.1530/EJE-21-0239
Colao A, Bronstein MD, Freda P et al (2014) Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 99(3):791–799. https://doi.org/10.1210/jc.2013-2480
Gadelha MR, Bronstein MD, Brue T et al (2014) Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2(11):875–884. https://doi.org/10.1016/S2213-8587(14)70169-X
Samson SL, Gu F, Feldt-Rasmussen U et al (2021) Managing pasireotide-associated hyperglycemia: a randomized, open-label Phase IV study. Pituitary 24(6):887–903. https://doi.org/10.1007/s11102-021-01161-4
Bolanowski M, Kaluzny M, Witek P et al (2022) Pasireotide-a novel somatostatin receptor ligand after 20 years of use. Rev Endocr Metab Disord 23(3):601–620. https://doi.org/10.1007/s11154-022-09710-3
Coopmans EC, Muhammad A, van der Lely AJ et al (2019) How to position pasireotide LAR treatment in acromegaly. J Clin Endocrinol Metab 104(6):1978–1988. https://doi.org/10.1210/jc.2018-01979
Bruns C, Lewis I, Briner U et al (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146(5):707–716. https://doi.org/10.1530/eje.0.1460707
Gatto F, Biermasz NR, Feelders RA et al (2016) Low beta-arrestin expression correlates with the responsiveness to long-term somatostatin analog treatment in acromegaly. Eur J Endocrinol 174(5):651–662. https://doi.org/10.1530/EJE-15-0391
Gatto F, Arvigo M, Amaru J et al (2019) Cell specific interaction of pasireotide: review of preclinical studies in somatotroph and corticotroph pituitary cells. Pituitary 22(1):89–99. https://doi.org/10.1007/s11102-018-0926-y
Poll F, Lehmann D, Illing S et al (2010) Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol 24(2):436–446. https://doi.org/10.1210/me.2009-0315
Gatto F, Barbieri F, Arvigo M et al (2019) Biological and biochemical basis of the differential efficacy of first and second generation somatostatin receptor ligands in neuroendocrine neoplasms. Int J Mol Sci. https://doi.org/10.3390/ijms20163940
Schmid HA (2008) Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol 286(1–2):69–74. https://doi.org/10.1016/j.mce.2007.09.006
Gadelha M, Bex M, Colao A et al (2019) Evaluation of the efficacy and safety of switching to pasireotide in patients with acromegaly inadequately controlled with first-generation somatostatin analogs. Front Endocrinol (Lausanne) 10:931. https://doi.org/10.3389/fendo.2019.00931
Colao A, Bronstein MD, Brue T et al (2020) Pasireotide for acromegaly: long-term outcomes from an extension to the Phase III PAOLA study. Eur J Endocrinol 182(6):583. https://doi.org/10.1530/EJE-19-0762
Gatto F, Feelders RA, Franck SE et al (2017) In vitro head-to-head comparison between octreotide and pasireotide in GH-secreting pituitary adenomas. J Clin Endocrinol Metab 102(6):2009–2018. https://doi.org/10.1210/jc.2017-00135
Lasolle H, Ferriere A, Vasiljevic A et al (2019) Pasireotide-LAR in acromegaly patients treated with a combination therapy: a real-life study. Endocr Connect 8(10):1383–1394. https://doi.org/10.1530/EC-19-0332
Witek P, Bolanowski M, Szamotulska K et al (2021) The effect of 6 months’ treatment with pasireotide LAR on glucose metabolism in patients with resistant acromegaly in real-world clinical settings. Front Endocrinol (Lausanne) 12:633944. https://doi.org/10.3389/fendo.2021.633944
Shimon I, Adnan Z, Gorshtein A et al (2018) Efficacy and safety of long-acting pasireotide in patients with somatostatin-resistant acromegaly: a multicenter study. Endocrine 62(2):448–455. https://doi.org/10.1007/s12020-018-1690-5
Stelmachowska-Banas M, Czajka-Oraniec I, Tomasik A et al (2022) Real-world experience with pasireotide-LAR in resistant acromegaly: a single center 1-year observation. Pituitary 25(1):180–190. https://doi.org/10.1007/s11102-021-01185-w
Muhammad A, van der Lely AJ, Delhanty PJD et al (2018) Efficacy and safety of switching to pasireotide in patients with acromegaly controlled with pegvisomant and first-generation somatostatin analogues (PAPE Study). J Clin Endocrinol Metab 103(2):586–595. https://doi.org/10.1210/jc.2017-02017
American Diabetes Association Professional Practice C, American Diabetes Association Professional Practice C, Practice C, Draznin B et al (2022) Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care 45(Suppl 1):S17–S38. https://doi.org/10.2337/dc22-S002
Giustina A, Barkhoudarian G, Beckers A et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21(4):667–678. https://doi.org/10.1007/s11154-020-09588-z
van Esdonk MJ, van Zutphen EJM, Roelfsema F et al (2018) How are growth hormone and insulin-like growth factor-1 reported as markers for drug effectiveness in clinical acromegaly research? A comprehensive methodologic review. Pituitary 21(3):310–322. https://doi.org/10.1007/s11102-018-0884-4
Fleseriu M, Dreval A, Bondar I et al (2022) Maintenance of response to oral octreotide compared with injectable somatostatin receptor ligands in patients with acromegaly: a phase 3, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 10(2):102–111. https://doi.org/10.1016/S2213-8587(21)00296-5
Fleseriu M, Molitch M, Dreval A et al (2021) Disease and treatment-related burden in patients with acromegaly who are biochemically controlled on injectable somatostatin receptor ligands. Front Endocrinol (Lausanne) 12:627711. https://doi.org/10.3389/fendo.2021.627711
Giustina A, Barkan A, Beckers A et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz096
Kasuki L, Wildemberg LE, Gadelha MR (2018) Management of endocrine disease: Personalized medicine in the treatment of acromegaly. Eur J Endocrinol 178(3):R89–R100. https://doi.org/10.1530/EJE-17-1006
Gadelha M, Marques NV, Fialho C et al (2023) Long-term efficacy and safety of pasireotide in patients with acromegaly: 14 years’ single-center real-world experience. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgad378
Mondin A, Manara R, Voltan G et al (2022) Pasireotide-induced shrinkage in GH and ACTH secreting pituitary adenoma: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 13:935759. https://doi.org/10.3389/fendo.2022.935759
Henry RR, Ciaraldi TP, Armstrong D et al (2013) Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab 98(8):3446–3453. https://doi.org/10.1210/jc.2013-1771
Gadelha MR, Gu F, Bronstein MD et al (2020) Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr Connect 9(12):1178–1190. https://doi.org/10.1530/EC-20-0361
Dal J, Rosendal C, Karmisholt J et al (2023) Sex difference in patients with controlled acromegaly-A multicentre survey. Clin Endocrinol (Oxf) 98(1):74–81. https://doi.org/10.1111/cen.14750
Pivonello R, Auriemma RS, Grasso LF et al (2017) Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary 20(1):46–62. https://doi.org/10.1007/s11102-017-0797-7
Giordano C, Ciresi A, Amato MC et al (2012) Clinical and metabolic effects of first-line treatment with somatostatin analogues or surgery in acromegaly: a retrospective and comparative study. Pituitary 15(4):539–551. https://doi.org/10.1007/s11102-011-0365-5
Reyes-Vidal C, Fernandez JC, Bruce JN et al (2014) Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J Clin Endocrinol Metab 99(11):4124–4132. https://doi.org/10.1210/jc.2014-2259
Caron PJ, Petersenn S, Houchard A et al (2017) Glucose and lipid levels with lanreotide autogel 120 mg in treatment-naive patients with acromegaly: data from the PRIMARYS study. Clin Endocrinol (Oxf) 86(4):541–551. https://doi.org/10.1111/cen.13285
Chen X, Shen G, Jiang J et al (2014) Pharmacokinetics and safety of subcutaneous pasireotide and intramuscular pasireotide long-acting release in Chinese male healthy volunteers: a phase I, single-center, open-label, randomized study. Clin Ther 36(8):1196–1210. https://doi.org/10.1016/j.clinthera.2014.06.006
Albani A, Ferrau F, Ciresi A et al (2018) Pasireotide treatment reduces cardiometabolic risk in Cushing’s disease patients: an Italian, multicenter study. Endocrine 61(1):118–124. https://doi.org/10.1007/s12020-018-1524-5
Simeoli C, Ferrigno R, De Martino MC et al (2020) The treatment with pasireotide in Cushing’s disease: effect of long-term treatment on clinical picture and metabolic profile and management of adverse events in the experience of a single center. J Endocrinol Invest 43(1):57–73. https://doi.org/10.1007/s40618-019-01077-8
Funding
No founds, grants, or other support were received for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Diego Ferone has been a speaker for and participated on advisory boards and received research grants from Novartis AAA, Ipsen, Camurus and Recordati. Diego Ferone is a member of the Editorial Board of Journal of Endocrinological Investigation. Annamaria Colao received research grants from Novo Nordisk and Ipsen. Rosario Pivonello has been the Principal Investigator of Clinical and/or Translational Research Studies for Novartis, HRA Pharma, Ipsen, Shire, Corcept Therapeutics, Cortendo AB, Janssen Cilag, Camurus, Strongbridge, and Pfizer; Co-investigator of Research Studies for Pfizer; received research grants from Novartis, Pfizer, Ipsen, HRA Pharma, Shire, IBSA, Strongbridge Biopharma; has been an occasional consultant for Novartis, Ipsen, Pfizer, Shire, HRA Pharma, Cortendo AB, Ferring, Strongbridge Biopharma, Recordati, Corcept Therapeutics, Crinetics Pharmaceuticals, ARH Healthcare, Biohealth Italia; and has received fees and honoraria for presentations from Novartis, Shire, Pfizer and Recordati beyond the confines of this work. The other Authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the Institutional ethical committees and with the 1964 Helsinki declarations and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1:
(PDF 542 KB)
Supplementary file 2:
(PDF 545 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Corica, G., Pirchio, R., Milioto, A. et al. Pasireotide effects on biochemical control and glycometabolic profile in acromegaly patients switched from combination therapies or unconventional dosages of somatostatin analogs. J Endocrinol Invest 47, 683–697 (2024). https://doi.org/10.1007/s40618-023-02186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02186-1