Abstract
Purpose
Nearly, 40% of the causes of male infertility remain idiopathic. The only suggested treatment in idiopathic oligo- and/or asthenozoospermia in normogonadotropic patients is the FSH. In the current clinical practice, efficacy is exclusively assessable through semen analysis after 3 months of treatment. No molecular markers of treatment efficacy are appliable in clinical practice. The aim of the present work is to evaluate the combination of extracellular signal regulated kinase (ERK) 1 and 2 and prolactin inducible peptide (PIP) as potential markers of idiopathic infertility and FSH treatment efficacy.
Methods
Western blot and confocal microscopy were performed to analyze the modulation of PIP and ERK1/2 in idiopathic infertile patients (IIP) sperm cells. Taking advantage of mass spectrometry analysis, we identified these proteins unequivocally in sperm cells.
Results
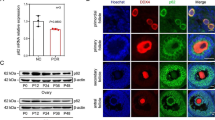
We demonstrated a significant decrease of both PIP protein and of ERK1/2 levels in spermatozoa obtained from IIP in comparison to healthy fertile patients (HFP). Conversely, we reported a significant increase of these markers comparing infertile patients before and after 3 months of FSH treatment. Importantly, this correlated with an increase in total number of sperm and sperm motility after FSH treatment. Finally, we identified of PIP and ERK2 proteins in sperm samples by proteomic analysis.
Conclusions
The combined evaluation of ERK1/2 and PIP proteins might represent a useful molecular marker to tailor FSH treatment in the management of male normogonadotropic idiopathic infertility.




Similar content being viewed by others
Data availability
Not applicable.
References
Vander Borght M, Wyns C (2018) Fertility and infertility: definition and epidemiology. Clin Biochem 62:2–10. https://doi.org/10.1016/J.CLINBIOCHEM.2018.03.012
Garolla A, Pizzol D, Carosso AR et al (2021) Practical clinical and diagnostic pathway for the investigation of the infertile couple. Front Endocrinol (Lausanne) 11:1032. https://doi.org/10.3389/FENDO.2020.591837/BIBTEX
Cousineau TM, Domar AD (2007) Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol 21:293–308. https://doi.org/10.1016/J.BPOBGYN.2006.12.003
Bourrion B, Panjo H, Bithorel PL et al (2022) The economic burden of infertility treatment and distribution of expenditures overtime in France: a self-controlled pre-post study. BMC Health Serv Res 22:1–10. https://doi.org/10.1186/S12913-022-07725-9/FIGURES/3
Kumar N, Singh A (2015) Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci 8:191. https://doi.org/10.4103/0974-1208.170370
Ventimiglia E, Pozzi E, Capogrosso P et al (2021) Extensive assessment of underlying etiological factors in primary infertile men reduces the proportion of men with idiopathic infertility. Front Endocrinol (Lausanne) 12:801125. https://doi.org/10.3389/fendo.2021.801125
Ferlin A, Calogero AE, Krausz C et al (2022) Management of male factor infertility: position statement from the Italian Society of andrology and sexual medicine (SIAMS): endorsing organization: Italian society of embryology, reproduction, and research (SIERR). J Endocrinol Invest 45:1085–1113. https://doi.org/10.1007/S40618-022-01741-6
Foresta C, Bettella A, Ferlin A et al (1998) Evidence for a stimulatory role of follicle-stimulating hormone on the spermatogonial population in adult males. Fertil Steril 69:636–642. https://doi.org/10.1016/S0015-0282(98)00008-9
Foresta C, Bettella A, Merico M et al (2002) Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil Steril 77:238–244. https://doi.org/10.1016/S0015-0282(01)02966-1
Arnaldi G, Balercia G, Barbatelli G, Mantero F (2000) Effects of long-term treatment with human pure follicle-stimulating hormone on semen parameters and sperm-cell ultrastructure in idiopathic oligoteratoasthenozoospermia. Andrologia 32:155–161. https://doi.org/10.1046/J.1439-0272.2000.00358.X
Ben-Rafael Z, Farhi J, Feldberg D et al (2000) Follicle-stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in vitro fertilization: the impact on sperm microstructure and fertilization potential. Fertil Steril 73:24–30. https://doi.org/10.1016/S0015-0282(99)00461-6
Muratori M, Baldi E (2018) Effects of FSH on sperm DNA fragmentation: review of clinical studies and possible mechanisms of action. Front Endocrinol (Lausanne) 9:734. https://doi.org/10.3389/FENDO.2018.00734
Santi D, Crépieux P, Reiter E et al (2020) Follicle-stimulating hormone (FSH) action on spermatogenesis: a focus on physiological and therapeutic roles. J Clin Med. https://doi.org/10.3390/JCM9041014
Simoni M, Brigante G, Rochira V et al (2020) Prospects for FSH Treatment of male infertility. J Clin Endocrinol Metab. https://doi.org/10.1210/CLINEM/DGAA243
Ponce MDR, Foresta C, Rago R et al (2020) Use of biosimilar follicle-stimulating hormone in asthenozoospermic infertile patients: a multicentric study. J Clin Med 9:1478. https://doi.org/10.3390/JCM9051478
Barbonetti A, Calogero AE, Balercia G et al (2018) The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J Endocrinol Invest. https://doi.org/10.1007/s40618-018-0843-y
Santi D, Granata ARM, Simoni M (2015) FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis. Endocr Connect 4:R46. https://doi.org/10.1530/EC-15-0050
Cannarella R, La Vignera S, Condorelli RA et al (2020) FSH dosage effect on conventional sperm parameters: a meta-analysis of randomized controlled studies. Asian J Androl 22:309–316. https://doi.org/10.4103/AJA.AJA_42_19
Attia AM, Abou-Setta AM, Al-Inany HG (2013) Gonadotrophins for idiopathic male factor subfertility. Cochrane database Syst Rev. https://doi.org/10.1002/14651858.CD005071.PUB4
Simoni M, Santi D, Negri L et al (2016) Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p. N680S: a pharmacogenetic study. Hum Reprod 31:1960–1969. https://doi.org/10.1093/HUMREP/DEW167
Grande G, Vincenzoni F, Mancini F et al (2019) Quantitative analysis of the seminal plasma proteome in secondary hypogonadism. J Clin Med 8:2128. https://doi.org/10.3390/JCM8122128
Hassan MI, Waheed A, Yadav S et al (2009) Prolactin inducible protein in cancer, fertility and immunoregulation: structure, function and its clinical implications. Cell Mol Life Sci 66:447–459. https://doi.org/10.1007/S00018-008-8463-X
Martínez-Heredia J, de Mateo S, Vidal-Taboada JM et al (2008) Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod 23:783–791. https://doi.org/10.1093/HUMREP/DEN024
Arthur JSC, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13:679–692. https://doi.org/10.1038/nri3495
Nan X, Tamgüney TM, Collisson EA et al (2015) Ras-GTP dimers activate the mitogen-activated protein kinase (MAPK) pathway. Proc Natl Acad Sci USA 112:7996–8001. https://doi.org/10.1073/PNAS.1509123112
Bianchetti G, Taralli S, Vaccaro M et al (2022) Automated detection and classification of tumor histotypes on dynamic PET imaging data through machine-learning driven voxel classification. Comput Biol Med. https://doi.org/10.1016/J.COMPBIOMED.2022.105423
Bianchetti G, Di Giacinto F, De Spirito M, Maulucci G (2020) Machine-learning assisted confocal imaging of intracellular sites of triglycerides and cholesteryl esters formation and storage. Anal Chim Acta 1121:57–66. https://doi.org/10.1016/J.ACA.2020.04.076
Berg S, Kutra D, Kroeger T et al (2019) ilastik: interactive machine learning for (bio)image analysis. Nat Methods 16:1226–1232. https://doi.org/10.1038/S41592-019-0582-9
Barbonetti A, Calogero AE, Balercia G et al (2018) The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: position statement from the Italian Society of andrology and sexual medicine (SIAMS). J Endocrinol Invest 41:1107–1122. https://doi.org/10.1007/S40618-018-0843-Y
Simoni M, Gromoll J, Nieschlag E (1997) The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18:739–773. https://doi.org/10.1210/EDRV.18.6.0320
Gloaguen P, Crépieux P, Heitzler D et al (2011) Mapping the follicle-stimulating hormone-induced signaling networks. Front Endocrinol (Lausanne). https://doi.org/10.3389/FENDO.2011.00045
Hunzicker-Dunn M, Maizels ET (2006) FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359. https://doi.org/10.1016/J.CELLSIG.2006.02.011
Conti AC, Cryan JF, Dalvi A et al (2002) cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci 22:3262–3268. https://doi.org/10.1523/JNEUROSCI.22-08-03262.2002
Casarini L, Crépieux P (2019) Molecular mechanisms of action of FSH. Front Endocrinol (Lausanne) 10:305. https://doi.org/10.3389/FENDO.2019.00305/BIBTEX
Garrido N, Martínez-Conejero JA, Jauregui J et al (2009) Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril 91:1307–1310. https://doi.org/10.1016/J.FERTNSTERT.2008.01.078
Bansal SK, Gupta N, Sankhwar SN, Rajender S (2015) Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS One. https://doi.org/10.1371/JOURNAL.PONE.0127007
Cui L, Fang L, Shi B et al (2018) Spermatozoa expression of piR-31704, piR-39888, and piR-40349 and their correlation to sperm concentration and fertilization rate after ICSI. Reprod Sci 25:733–739. https://doi.org/10.1177/1933719117725822/METRICS
Agarwal A, Parekh N, Selvam MKP et al (2019) Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health 37:296. https://doi.org/10.5534/WJMH.190055
Bruno C, Basile U, Vergani E et al (2022) Inflammation and oxidative stress in seminal plasma: search for biomarkers in diagnostic approach to male infertility. J Pers Med. https://doi.org/10.3390/JPM12060857
Mongioì LM, Condorelli RA, Alamo A et al (2020) Follicle-stimulating hormone treatment and male idiopathic infertility: effects on sperm parameters and oxidative stress indices according to FSHR c. 2039 A/G and c. -29 G/A genotypes. J Clin Med. https://doi.org/10.3390/JCM9061690
Milardi D, Grande G, Vincenzoni F et al (2014) Novel biomarkers of androgen deficiency from seminal plasma profiling using high-resolution mass spectrometry. J Clin Endocrinol Metab 99:2813–2820. https://doi.org/10.1210/JC.2013-4148
Buscà R, Pouysségur J, Lenormand P (2016) ERK1 and ERK2 map kinases: Specific roles or functional redundancy? Front Cell Dev Biol 4:53. https://doi.org/10.3389/FCELL.2016.00053/BIBTEX
Almog T, Lazar S, Reiss N et al (2008) Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J Biol Chem 283:14479–14489. https://doi.org/10.1074/JBC.M710492200
Almog T, Naor Z (2008) Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Mol Cell Endocrinol 282:39–44. https://doi.org/10.1016/J.MCE.2007.11.011
Almog T, Naor Z (2010) The role of mitogen activated protein kinase (MAPK) in sperm functions. Mol Cell Endocrinol 314:239–243. https://doi.org/10.1016/J.MCE.2009.05.009
Li MWM, Mruk DD, Cheng CY (2009) Mitogen-activated protein kinases in male reproductive function. Trends Mol Med 15:159–168. https://doi.org/10.1016/J.MOLMED.2009.02.002
Dahia CL, Rao AJ (2006) Demonstration of follicle-stimulating hormone receptor in cauda epididymis of rat. Biol Reprod 75:98–106. https://doi.org/10.1095/BIOLREPROD.105.047704
Świder-Al-Amawi M, Kolasa A, Sikorski A et al (2010) The immunoexpression of FSH-R in the ductuli efferentes and the epididymis of men and rat: effect of FSH on the morphology and steroidogenic activity of rat epididymal epithelial cells in vitro. J Biomed Biotechnol. https://doi.org/10.1155/2010/506762
Sullivan R (2016) Epididymosomes: role of extracellular microvesicles in sperm maturation. Front Biosci (Schol Ed) 8:106–114. https://doi.org/10.2741/S450
Sullivan R, Mieusset R (2016) The human epididymis: its function in sperm maturation. Hum Reprod Update 22:574–587. https://doi.org/10.1093/HUMUPD/DMW015
da Juliano SC, de Ávila ACFCM, Garrett HL et al (2018) Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol 236:R15–R27. https://doi.org/10.1530/JOE-17-0200
Recchia K, Jorge AS, de Pessôa FLV et al (2021) Actions and roles of FSH in germinative cells. Int J Mol Sci 22:10110. https://doi.org/10.3390/IJMS221810110
Baccetti B, Collodel G, Costantino-Ceccarini E et al (1998) Localization of human follicle-stimulating hormone in the testis. FASEB J 12:1045–1054. https://doi.org/10.1096/FASEBJ.12.11.1045
Panza S, Giordano F, De Rose D et al (2020) FSH-R human early male genital tract, testicular tumors and sperm: its involvement in testicular disorders. Life 10:1–19. https://doi.org/10.3390/LIFE10120336
Acknowledgements
We thank Alessia Mahoney, University of Cardiff, Cardiff, United Kingdom for her kind and careful English editing.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, FM, FDN and DM; methodology, FM, FDN and ET; validation, GB, FI, DM and FM; formal analysis, GB, FI and ET; investigation, FM, FDN; data curation, FM, FI, GG and FDN; writing—original draft preparation, FM, FDN, EV and DM; writing—review and editing, CB, EV, ET and AU; visualization, AP, AU, GM, GG and MDS; supervision, DM, AU and AP. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
The study was carried out following the principles and the guidelines of the Declaration of Helsinki (version 2013) and all subjects gave written.
Informed consent
Sperm collection and analysis was approved by the Fondazione Policlinico Universitario A. Gemelli, IRCCS, local ethics committee (ID 3943).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mancini, F., Di Nicuolo, F., Teveroni, E. et al. Combined evaluation of prolactin-induced peptide (PIP) and extracellular signal-regulated kinase (ERK) as new sperm biomarkers of FSH treatment efficacy in normogonadotropic idiopathic infertile men. J Endocrinol Invest 47, 455–468 (2024). https://doi.org/10.1007/s40618-023-02161-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02161-w