Abstract
Purpose
Thyroid cell lines are useful tools to study the physiology and pathology of the thyroid, however, they do not produce or secrete hormones in vitro. On the other hand, the detection of endogenous thyroid hormones in primary thyrocytes was often hindered by the dedifferentiation of thyrocytes ex vivo and the presence of large amounts of exogenous hormones in the culture medium. This study aimed to create a culture system that could maintain the function of thyrocytes to produce and secrete thyroid hormones in vitro.
Methods
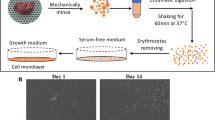
We established a Transwell culture system of primary human thyrocytes. Thyrocytes were seeded on a porous membrane in the inner chamber of the Transwell with top and bottom surfaces exposed to different culture components, mimicking the ‘lumen-capillary’ structure of the thyroid follicle. Moreover, to eliminate exogenous thyroid hormones from the culture medium, two alternatives were tried: a culture recipe using hormone-reduced serum and a serum-free culture recipe.
Results
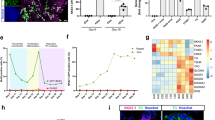
The results showed that primary human thyrocytes expressed thyroid-specific genes at higher levels in the Transwell system than in the monolayer culture. Hormones were detected in the Transwell system even in the absence of serum. The age of the donor was negatively related to the hormone production of thyrocytes in vitro. Intriguingly, primary human thyrocytes cultured without serum secreted higher levels of free triiodothyronine (FT3) than free thyroxine (FT4).
Conclusion
This study confirmed that primary human thyrocytes could maintain the function of hormone production and secretion in the Transwell system, thus providing a useful tool to study thyroid function in vitro.






Similar content being viewed by others
Data availability
All data will be available upon requests directed to the corresponding authors.
References
Pirahanchi Y, Tariq MA, Jialal I (2022) “Physiology, Thyroid," in StatPearls. Treasure Island (FL)
Hegedus L, Bianco AC, Jonklaas J, Pearce SH, Weetman AP, Perros P (2022) Primary hypothyroidism and quality of life. Nat Rev Endocrinol 18(4):230–242. https://doi.org/10.1038/s41574-021-00625-8
Blick C, Nguyen M, Jialal I (2022) "Thyrotoxicosis," in StatPearls. Treasure Island (FL)
Grasberger H, Refetoff S (2006) Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281(27):18269–18272. https://doi.org/10.1074/jbc.C600095200
Rousset B, Dupuy C, Miot F, Dumont J (2000) "Chapter 2 Thyroid Hormone Synthesis And Secretion," In: Feingold KR, Anawalt B, Boyce A, et al. (Eds.) Endotext. South Dartmouth (MA)
Saiselet M, Floor S, Tarabichi M, Dom G, Hebrant A, van Staveren WC et al (2012) Thyroid cancer cell lines: an overview. Front Endocrinol (Lausanne). 3:133. https://doi.org/10.3389/fendo.2012.00133
Ishido Y, Yamazaki K, Kammori M, Sugishita Y, Luo Y, Yamada E et al (2014) Thyroglobulin suppresses thyroid-specific gene expression in cultures of normal but not neoplastic human thyroid follicular cells. J Clin Endocrinol Metab 99(4):E694-702. https://doi.org/10.1210/jc.2013-3682
Shi H, Lin W, Liang BO, Cai H, Cai Q, Shi Y et al (2016) Presence of free triiodothyronine and free thyroxine in thyroid follicles may be correlated with the quick secretion of thyroid hormones under certain physiological conditions. Biomed Rep 4(4):467–470. https://doi.org/10.3892/br.2016.596
Bravo SB, Garcia-Rendueles ME, Garcia-Rendueles AR, Rodrigues JS, Perez-Romero S, Garcia-Lavandeira M et al (2013) Humanized medium (h7H) allows long-term primary follicular thyroid cultures from human normal thyroid, benign neoplasm, and cancer. J Clin Endocrinol Metab 98(6):2431–2441. https://doi.org/10.1210/jc.2012-3812
Jang D, Marcus-Samuels B, Morgan SJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC (2020) Thyrotropin regulation of differentiated gene transcription in adult human thyrocytes in primary culture. Mol Cell Endocrinol. 518:111032. https://doi.org/10.1016/j.mce.2020.111032
Nilsson M, Husmark J, Nilsson B, Tisell LE, Ericson LE (1996) Primary culture of human thyrocytes in Transwell bicameral chamber: thyrotropin promotes polarization and epithelial barrier function. Eur J Endocrinol 135(4):469–480. https://doi.org/10.1530/eje.0.1350469
Uyttersprot N, Costagliola S, Miot F (1998) A new tool for efficient transfection of dog and human thyrocytes in primary culture. Mol Cell Endocrinol 142(1–2):35–39. https://doi.org/10.1016/s0303-7207(98)00122-1
Takasu N, Ohno S, Komiya I, Yamada T (1992) Requirements of follicle structure for thyroid hormone synthesis; cytoskeletons and iodine metabolism in polarized monolayer cells on collagen gel and in double layered, follicle-forming cells. Endocrinology 131(3):1143–1148. https://doi.org/10.1210/endo.131.3.1505456
Toda S, Koike N, Sugihara H (2001) Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: perspective for thyroid tissue engineering. Pathol Int 51(6):403–417. https://doi.org/10.1046/j.1440-1827.2001.01218.x
Toda S, Watanabe K, Yokoi F, Matsumura S, Suzuki K, Ootani A et al (2002) A new organotypic culture of thyroid tissue maintains three-dimensional follicles with C cells for a long term. Biochem Biophys Res Commun 294(4):906–911. https://doi.org/10.1016/S0006-291X(02)00561-2
Toda S, Aoki S, Suzuki K, Koike E, Ootani A, Watanabe K et al (2003) Thyrocytes, but not C cells, actively undergo growth and folliculogenesis at the periphery of thyroid tissue fragments in three-dimensional collagen gel culture. Cell Tissue Res 312(3):281–289. https://doi.org/10.1007/s00441-003-0718-0
Toda S, Aoki S, Uchihashi K, Matsunobu A, Yamamoto M, Ootani A et al (2011) Culture models for studying thyroid biology and disorders. ISRN Endocrinol. https://doi.org/10.5402/2011/275782
Samuels HH, Stanley F, Casanova J (1979) Depletion of L-3,5,3’-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105(1):80–85. https://doi.org/10.1210/endo-105-1-80
Back CM, Stohr S, Schafer EA, Biebermann H, Boekhoff I, Breit A et al (2013) TSH induces metallothionein 1 in thyrocytes via Gq/11- and PKC-dependent signaling. J Mol Endocrinol 51(1):79–90. https://doi.org/10.1530/JME-12-0200
Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A et al (2011) A Signaling network of thyroid-stimulating hormone. J Proteomics Bioinform. https://doi.org/10.4172/jpb.1000195
Sellitti DF, Suzuki K (2014) Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 24(4):625–638. https://doi.org/10.1089/thy.2013.0344
Wang Y, Li W, Phay JE, Shen R, Pellegata NS, Saji M et al (2016) Primary cell culture systems for human thyroid studies. Thyroid 26(8):1131–1140. https://doi.org/10.1089/thy.2015.0518
Morgan SJ, Neumann S, Marcus-Samuels B, Gershengorn MC (2016) Thyrotropin and insulin-like growth factor 1 receptor crosstalk upregulates sodium-iodide symporter expression in primary cultures of human thyrocytes. Thyroid 26(12):1794–1803. https://doi.org/10.1089/thy.2016.0323
Lorenz S, Aust G, Krohn K (2013) Ca(2+)-binding protein expression in primary human thyrocytes. Biochim Biophys Acta 1833(12):2703–2713. https://doi.org/10.1016/j.bbamcr.2013.07.014
Liang ZR, Qu LH, Ma LM (2020) Differential impacts of charcoal-stripped fetal bovine serum on c-Myc among distinct subtypes of breast cancer cell lines. Biochem Biophys Res Commun 526(1):267–272. https://doi.org/10.1016/j.bbrc.2020.03.049
Tu C, Fiandalo MV, Pop E, Stocking JJ, Azabdaftari G, Li J et al (2018) Proteomic Analysis of Charcoal-stripped fetal bovine serum reveals changes in the insulin-like growth factor signaling pathway. J Proteome Res 17(9):2963–2977. https://doi.org/10.1021/acs.jproteome.8b00135
Mishra V, Heath RJ (2021) Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int J Mol Sci. https://doi.org/10.3390/ijms22168411
Baral S, Shrestha PK, Pant V (2017) Serum free T3 to free T4 ratio as a useful indicator for differentiating destruction induced thyrotoxicosis from Graves’ disease. J Clin Diagn Res. 11(7):OC12–OC14. https://doi.org/10.7860/JCDR/2017/28293.10180
Moreno M, Berry MJ, Horst C, Thoma R, Goglia F, Harney JW et al (1994) Activation and inactivation of thyroid hormone by type I iodothyronine deiodinase. FEBS Lett 344(2–3):143–146. https://doi.org/10.1016/0014-5793(94)00365-3
Yamazaki K, Suzuki K, Yamada E, Yamada T, Takeshita F, Matsumoto M et al (2007) Suppression of iodide uptake and thyroid hormone synthesis with stimulation of the type I interferon system by double-stranded ribonucleic acid in cultured human thyroid follicles. Endocrinology 148(7):3226–3235. https://doi.org/10.1210/en.2006-1638
Kawashima A, Tanigawa K, Akama T, Wu H, Sue M, Yoshihara A et al (2011) Fragments of genomic DNA released by injured cells activate innate immunity and suppress endocrine function in the thyroid. Endocrinology 152(4):1702–1712. https://doi.org/10.1210/en.2010-1132
Acknowledgements
The authors thank Professor Koichi Suzuki (Teikyo University, Tokyo, Japan) sincerely for his generous advice during the progression of this study.
Funding
This work was supported by the National Natural Science Foundation of China #81900712.
Author information
Authors and Affiliations
Contributions
BJ and CW contributed to this work equally. LS, and YL contributed to the study concept and design. BJ, CQ, CJ, and CZ contributed to the acquisition of data (sample collection, processing, performance of experiment, etc.). CW, YC, and FC contributed to the analysis, interpretation, and statistical analysis. YL and CW contributed to the drafting of the manuscript. LS and FC contributed to the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethical Committee of the institutional review boards of Nanjing Drum Tower Hospital, Nanjing, China (No. 2021-601-02).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40618_2023_2103_MOESM1_ESM.pdf
Supplementary file1 (PDF 52 KB) Supplementary Fig. 1 Transwell system and monolayer culture of primary human thyrocytes. (a) In the Transwell system, primary human thyrocytes were seeded on a porous membrane of the inner chamber. After cells reached 100% confluency, the inner chamber was refilled with DMEM while the outer chamber with DMEM supplemented with 10% FBS or otherwise indicated. Thus, the up and bottom surfaces of primary human thyrocytes, mimicking the apical and basal sides, could access different culture components. (b) In a conventional monolayer culture, the primary human thyrocytes has only one surface unattached that was exposed to DMEM supplemented with 10% FBS. (c) For analysis of thyroid-specific genes, primary human thyrocytes were seeded in Transwell system using DMEM supplemented with 10% FBS and cultured for 6 days. On day 7, the inner chamber was refilled with DMEM, and the outer chamber with DMEM supplemented with 10% FBS. (d) For analysis of thyroid hormones, primary human thyrocytes were seeded in Transwell system using DMEM supplemented with 10% FBS and cultured for 6 days. On day 7, the inner chamber was refilled with DMEM, and the outer chamber with the cFBS-formulated medium or the rHSA-formulated medium (details in Materials and Methods). The medium of the outer chamber was then collected on day 10 and day 14 and subjected to hormone analysis. Supplementary Fig. 2 The workflow of embedding and cryostat of the porous membrane with cultured primary human thyrocytes. The porous membrane (on which the primary human thyrocytes were seeded) of Transwell was carefully removed from the inner chamber and dehydrated. For dehydration, the membrane was first placed in 30% sucrose at 4°C for overnight and then in a mixture of 30% sucrose and cryostat embedding media OCT (1:1) at room temperature for 2h. The dehydrated membrane was then cut into pieces and embedded in OCT in a vertical position followed by cryostat of a transverse plane
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, B., Wang, C., Qu, C. et al. Primary human thyrocytes maintained the function of thyroid hormone production and secretion in vitro. J Endocrinol Invest 46, 2501–2512 (2023). https://doi.org/10.1007/s40618-023-02103-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02103-6