Abstract
Background
Thyroid dysfunction is among the most common immune-related adverse events (irAEs) of immune checkpoint inhibitors (ICIs) therapy. Data regarding potential predictors of the development of thyroid irAEs are still limited and sometimes conflicting.
Patients and methods
We assessed potential risk factors and clinical outcomes associated with the onset of thyroid irAEs in a cohort of patients with different types of cancer treated with ICIs at a single center. Clinical and biochemical data, including thyroid function tests and autoantibodies at baseline and during treatment, were collected, and the onset of thyroid irAEs was recorded. Patients with thyroid dysfunction and/or under levothyroxine therapy before starting ICI were excluded.
Results
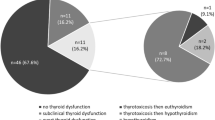
110 patients (80 M, 30 F, aged 32–85 years; 56.4% non-small-cell lung cancer, 87% treated with anti-PD-1) with complete information were included in the study. Among them, 32 (29%) developed thyroid irAEs during ICIs therapy. Primary hypothyroidism was the most common irAEs, occurring in 31 patients (28.18% of the whole cohort), including 14 patients who experienced a transient thyrotoxicosis. About 60% of irAEs occurred within the first 8 weeks of therapy. At multivariate analysis, anti-thyroid autoantibodies positivity at baseline (OR 18.471, p = 0.022), a pre-existing (autoimmune and non-autoimmune) thyroid disorder (OR 16.307, p < 0.001), and a family history of thyroid diseases (OR = 9.287, p = 0.002) were independent predictors of the development of thyroid irAEs.
Conclusion
Our data confirm the high frequency of thyroid dysfunctions (mostly hypothyroidism) during ICIs, and provide data on valuable predictors of thyroid toxicities that may help clinicians in identifying patients at risk for developing irAEs.

Similar content being viewed by others
References
Stewart TJ, Smyth MJ (2011) Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev 30:125–140. https://doi.org/10.1007/s10555-011-9280-5
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Twomey JD, Zhang B (2021) Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J 23:39. https://doi.org/10.1208/s12248-021-00574-0
Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob JJ, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, Li B, Hodi FS, Long GV, Investigators R (2022) Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 386:24–34. https://doi.org/10.1056/NEJMoa2109970
Spagnolo CC, Giuffrida G, Cannavo S, Franchina T, Silvestris N, Ruggeri RM, Santarpia M (2022) Management of endocrine and metabolic toxicities of immune-checkpoint inhibitors: from clinical studies to a real-life scenario. Cancers (Basel). https://doi.org/10.3390/cancers15010246
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982. https://doi.org/10.1200/JCO.2014.59.4358
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168. https://doi.org/10.1056/NEJMra1703481
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM (2018) Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4:173–182. https://doi.org/10.1001/jamaoncol.2017.3064
Inno A, Metro G, Bironzo P, Grimaldi AM, Grego E, Di Nunno V, Picasso V, Massari F, Gori S (2017) Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori 103:405–421. https://doi.org/10.5301/tj.5000625
Del Rivero J, Cordes LM, Klubo-Gwiezdzinska J, Madan RA, Nieman LK, Gulley JL (2020) Endocrine-related adverse events related to immune checkpoint inhibitors: proposed algorithms for management. Oncologist 25:290–300. https://doi.org/10.1634/theoncologist.2018-0470
Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L (2019) Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 40:17–65. https://doi.org/10.1210/er.2018-00006
Ruggeri RM, Campenni A, Giuffrida G, Trimboli P, Giovanella L, Trimarchi F, Cannavo S (2019) Endocrine and metabolic adverse effects of immune checkpoint inhibitors: an overview (what endocrinologists should know). J Endocrinol Invest 42:745–756. https://doi.org/10.1007/s40618-018-0984-z
Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM (2021) Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab 106:e3704–e3713. https://doi.org/10.1210/clinem/dgab263
von Itzstein MS, Gonugunta AS, Wang Y, Sheffield T, Lu R, Ali S, Fattah FJ, Xie D, Cai J, Xie Y, Gerber DE (2022) Divergent prognostic effects of pre-existing and treatment-emergent thyroid dysfunction in patients treated with immune checkpoint inhibitors. Cancer Immunol Immunother 71:2169–2181. https://doi.org/10.1007/s00262-022-03151-2
Muir CA, Tsang VHM, Menzies AM, Clifton-Bligh RJ (2022) Immune related adverse events of the thyroid—a narrative review. Front Endocrinol (Lausanne) 13:886930. https://doi.org/10.3389/fendo.2022.886930
Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, Busaidy NL, Subudhi SK, Diab A, Dadu R (2018) Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 28:1243–1251. https://doi.org/10.1089/thy.2018.0116
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894. https://doi.org/10.1158/1078-0432.CCR-15-1136
Presotto EM, Rastrelli G, Desideri I, Scotti V, Gunnella S, Pimpinelli N, Vaccher E, Bearz A, Di Costanzo F, Bruggia M, Mini E, Maggi M, Peri A (2020) Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab: results of a large multicentre study. J Endocrinol Invest 43:337–345. https://doi.org/10.1007/s40618-019-01112-8
Percik R, Shoenfeld Y (2020) Check point inhibitors and autoimmunity: Why endocrinopathies and who is prone to? Best Pract Res Clin Endocrinol Metab 34:101411. https://doi.org/10.1016/j.beem.2020.101411
Rubino R, Marini A, Roviello G, Presotto EM, Desideri I, Ciardetti I, Brugia M, Pimpinelli N, Antonuzzo L, Mini E, Livi L, Maggi M, Peri A (2021) Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine 74:172–179. https://doi.org/10.1007/s12020-021-02750-w
Peiffert M, Cugnet-Anceau C, Dalle S, Chikh K, Assaad S, Disse E, Raverot G, Borson-Chazot F, Abeillon-du Payrat J (2021) Graves’ disease during immune checkpoint inhibitor therapy (a case series and literature review). Cancers (Basel). https://doi.org/10.3390/cancers13081944
Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, Larkin J, Peters S, Thompson JA, Obeid M (2020) Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 31:724–744. https://doi.org/10.1016/j.annonc.2020.03.285
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J (2017) Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 86:614–620. https://doi.org/10.1111/cen.13297
Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH (2018) The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol 178:173–180. https://doi.org/10.1530/EJE-17-0810
Guaraldi F, La Selva R, Sama MT, D’Angelo V, Gori D, Fava P, Fierro MT, Savoia P, Arvat E (2018) Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. J Endocrinol Invest 41:549–556. https://doi.org/10.1007/s40618-017-0772-1
Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M (2017) Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab 102:2770–2780. https://doi.org/10.1210/jc.2017-00448
Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, Kobayashi T, Tsuji T, Minomo S, Tamiya A, Atagi S (2017) Predictive factors of nivolumab-induced hypothyroidism in patients with non-small cell lung cancer. In Vivo 31:1035–1039. https://doi.org/10.21873/invivo.11166
Luongo C, Morra R, Gambale C, Porcelli T, Sessa F, Matano E, Damiano V, Klain M, Schlumberger M, Salvatore D (2021) Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest 44:1927–1933. https://doi.org/10.1007/s40618-021-01508-5
Basak EA, van der Meer JWM, Hurkmans DP, Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, Bins S, Joosse A, Koolen SLW, Debets R, Peeters RP, Aerts J, Mathijssen RHJ, Medici M (2020) Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid 30:966–973. https://doi.org/10.1089/thy.2019.0726
Brilli L, Danielli R, Campanile M, Secchi C, Ciuoli C, Calabro L, Pilli T, Cartocci A, Pacini F, Di Giacomo AM, Castagna MG (2021) Baseline serum TSH levels predict the absence of thyroid dysfunction in cancer patients treated with immunotherapy. J Endocrinol Invest 44:1719–1726. https://doi.org/10.1007/s40618-020-01480-6
Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, Ito M, Okada N, Enomoto A, Ito S, Yan Y, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Goto M, Suga H, Banno R, Takahashi M, Nishikawa H, Arima H (2021) CD4(+) T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abb7495
Gutzmer R, Koop A, Meier F, Hassel JC, Terheyden P, Zimmer L, Heinzerling L, Ugurel S, Pfohler C, Gesierich A, Livingstone E, Satzger I, Kahler KC, German Dermatooncology G (2017) Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 75:24–32. https://doi.org/10.1016/j.ejca.2016.12.038
Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, Azimi R, Rizvi H, Riess JW, Hellmann MD, Awad MM (2018) Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 36:1905–1912. https://doi.org/10.1200/JCO.2017.77.0305
Kahler KC, Eigentler TK, Gesierich A, Heinzerling L, Loquai C, Meier F, Meiss F, Pfohler C, Schlaak M, Terheyden P, Thoms KM, Ziemer M, Zimmer L, Gutzmer R, German Dermatologic Cooperative Oncology G (2018) Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother 67:825–834. https://doi.org/10.1007/s00262-018-2134-z
Hoa S, Laaouad L, Roberts J, Ennis D, Ye C, Al Jumaily K, Pope J, Nevskaya T, Saltman A, Himmel M, Rottapel R, Ly C, Colmegna I, Fifi-Mah A, Maltez N, Tisseverasinghe A, Hudson M, Jamal S (2021) Preexisting autoimmune disease and immune-related adverse events associated with anti-PD-1 cancer immunotherapy: a national case series from the Canadian research group of rheumatology in immuno-oncology. Cancer Immunol Immunother 70:2197–2207. https://doi.org/10.1007/s00262-021-02851-5
Ghosh N, Chan KK, Jivanelli B, Bass AR (2022) Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors: a systematic literature review. J Clin Rheumatol 28:e498–e505. https://doi.org/10.1097/RHU.0000000000001777
Iadarola C, Croce L, Quaquarini E, Teragni C, Pinto S, Bernardo A, Fonte R, Marinò M, Rotondi M, Chiovato L (2019) Nivolumab induced thyroid dysfunction: unusual clinical presentation and challenging diagnosis. Front Endocrinol (Lausanne) 9:813. https://doi.org/10.3389/fendo.2018.00813
Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, Otsuka A, Yamasaki T, Saito R, Kitamura M, Kitawaki T, Hishizawa M, Kawaguchi-Sakita N, Fujii T, Taura D, Sone M, Inagaki N (2019) Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 14:e0216954. https://doi.org/10.1371/journal.pone.0216954
Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, Takeshima K, Furukawa Y, Morita S, Yamamoto Y, Yamashita S, Katsuda M, Hayata A, Akamatsu H, Jinnin M, Hara I, Yamaue H, Akamizu T (2020) Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 111:1468–1477. https://doi.org/10.1111/cas.14363
Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, Jeon MJ, Kim TY, Kim SW, Kim WB, Kim SW, Lee DH, Park K, Ahn MJ, Chung JH, Shong YK, Kim WG, Kim TH (2017) Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 7:e1375642. https://doi.org/10.1080/2162402X.2017.1375642
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Kotwal A, Kottschade L, Ryder M (2020) PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 30:177–184. https://doi.org/10.1089/thy.2019.0250
Lima Ferreira J, Costa C, Marques B, Castro S, Victor M, Oliveira J, Santos AP, Sampaio IL, Duarte H, Marques AP, Torres I (2021) Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother 70:299–309. https://doi.org/10.1007/s00262-020-02664-y
Baldini E, Lunghi A, Cortesi E, Turci D, Signorelli D, Stati V, Melotti B, Ricciuti B, Frassoldati A, Romano G, Ceresoli GL, Illiano A, Verderame F, Fasola G, Ricevuto E, Marchetti P, Pinto C, Carteni G, Scotti V, Tibaldi C, Fioretto L, Giannarelli D (2020) Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: the Italian NSCLC expanded access program. Lung Cancer 140:59–64. https://doi.org/10.1016/j.lungcan.2019.12.014
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145:479–485. https://doi.org/10.1007/s00432-018-2805-3
Hsiehchen D, Naqash AR, Espinoza M, Von Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y, Burke M, Xie Y, Gerber DE (2022) Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology 11:2017162. https://doi.org/10.1080/2162402X.2021.2017162
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Patients were treated and followed according to international guidelines and local practices. Local Ethics Research Committee approval was obtained. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee. This study complies with the Declaration of Helsinki.
Research involving human participants and/or animals
All procedures were approved by he Local Ethics Research Committee of the University Hospital Policlinico “G. Martino”. The study was carried out in accordance with the World Medical Association’s Declaration of Helsinki. No animals were used for this study.
Informed consent
Written informed consent was obtained from the patients for publication of this case series.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruggeri, R.M., Spagnolo, C.C., Alibrandi, A. et al. Predictors of thyroid adverse events during cancer immunotherapy: a real-life experience at a single center. J Endocrinol Invest 46, 2399–2409 (2023). https://doi.org/10.1007/s40618-023-02096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02096-2