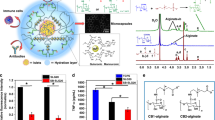
Abstract
Purpose
Hypoparathyroidism is a rare disease with low PTH, mostly seen as a consequence of neck surgery. Current management is the prescription of calcium and vitamin D, but the definitive treatment is parathyroid allotransplantation, which frequently triggers an immune response, thus cannot achieve the expected success. To overcome this problem, encapsulation of allogeneic cells is the most promising method. By optimizing the standard alginate cell encapsulation technique with parathyroid cells under high-voltage application, the authors reduced the size of parathyroid-encapsulated beads and evaluated these samples in vitro and in vivo.
Methods
Parathyroid cells were isolated, and standard-sized alginate macrobeads were prepared without any electrical field application, while microbeads in smaller sizes (< 500 µm), by the application of 13 kV. Bead morphologies, cell viability, and PTH secretion were evaluated in vitro for four weeks. For the in vivo part, beads were transplanted into Sprague–Dawley rats, and after retrieval, immunohistochemistry and PTH release were evaluated in addition to the assessment of cytokine/chemokine levels.
Results
The viability of parathyroid cells in micro- and macrobeads did not differ significantly. However, the amount of in vitro PTH secretion from microencapsulated cells was significantly lower than that from macroencapsulated cells, although it increased throughout the incubation period. Immunohistochemistry of PTH staining in both of the encapsulated cells identified as positive after retrieval.
Conclusion
Contrary to the literature, a minimal in vivo immune response was developed for alginate-encapsulated parathyroid cells, regardless of bead size. Our findings suggest that injectable, micro-sized beads obtained using high-voltage may be a promising method for a non-surgical transplantation approach.
Graphical abstract









Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Bilezikian JP (2020) Hypoparathyroidism. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa113
Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ 3rd, Shaha AR, Shindo ML, Snyder SK, Stack BC Jr, Sunwoo JB, Wang MB (2018) American thyroid association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 28:830–841. https://doi.org/10.1089/thy.2017.0309
Konca Degertekin C, Gogas Yavuz D, Pekkolay Z, Saygili E, Ugur K, Or Koca A, Unubol M, Topaloglu O, Aydogan BI, Ozdemir Kutbay N, Hekimsoy Z, Yilmaz N, Balci MK, Tanrikulu S, Aydogan Unsal Y, Ersoy C, Omma T, Keskin M, Yalcin MM, Eroglu M (2022) Identifying clinical characteristics of hypoparathyroidism in turkey: HIPOPARATURK-NET study. Calcif Tissue Int 110:204–214. https://doi.org/10.1007/s00223-021-00908-2
Cipriani C, Pepe J, Biamonte F, Manai R, Biondi P, Nieddu L, Cianferotti L, Brandi ML, Minisola S (2017) The epidemiology of hypoparathyroidism in Italy: an 8-year register-based study. Calcif Tissue Int 100:278–285. https://doi.org/10.1007/s00223-016-0222-7
Clarke BL, Brown EM, Collins MT, Juppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV (2016) Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 101:2284–2299. https://doi.org/10.1210/jc.2015-3908
Astor MC, Lovas K, Debowska A, Eriksen EF, Evang JA, Fossum C, Fougner KJ, Holte SE, Lima K, Moe RB, Myhre AG, Kemp EH, Nedrebo BG, Svartberg J, Husebye ES (2016) Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J Clin Endocrinol Metab 101:3045–3053. https://doi.org/10.1210/jc.2016-1477
Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, Thakker RV, Khan AA, Potts JT Jr (2016) Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 101:2273–2283. https://doi.org/10.1210/jc.2015-3907
Zhang JLH, Appelman-Dijkstra NM, Schepers A (2022) Parathyroid allotransplantation: a systematic review. Med Sci 10:19
Cabane P, Gac P, Amat J, Pineda P, Rossi R, Caviedes R, Caviedes P (2009) Allotransplant of microencapsulated parathyroid tissue in severe postsurgical hypoparathyroidism: a case report. Transplant Proc 41:3879–3883. https://doi.org/10.1016/j.transproceed.2009.06.211
Flechner SM, Berber E, Askar M, Stephany B, Agarwal A, Milas M (2010) Allotransplantation of cryopreserved parathyroid tissue for severe hypocalcemia in a renal transplant recipient. Am J Transplant 10:2061–2065. https://doi.org/10.1111/j.1600-6143.2010.03234.x
Pareta R, McQuilling JP, Sittadjody S, Jenkins R, Bowden S, Orlando G, Farney AC, Brey EM, Opara EC (2014) Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas 43:605–613. https://doi.org/10.1097/MPA.0000000000000107
Ueng SW, Lee MS, Lin SS, Chan EC, Liu SJ (2007) Development of a biodegradable alginate carrier system for antibiotics and bone cells. J Orthop Res 25:62–72
Hsu BR, Chen HC, Fu SH, Huang YY, Huang HS (1994) The use of field effects to generate calcium alginate microspheres and its application in cell transplantation. J Formos Med Assoc 93:240–245
Rabanel JM, Banquy X, Zouaoui H, Mokhtar M, Hildgen P (2009) Progress technology in microencapsulation methods for cell therapy. Biotechnol Prog 25:946–963
Zhou Y, Si K, Masuhara H, Hosokawa Y, Kaji T, Fukui K (2009) A new size and shape controlling method for producing calcium alginate beads with immobilized proteins. J Biomed Sci Eng 2:287
Prüße U, Jahnz U, Wittlich P, Breford J, Vorlop K-D (2002) Bead production with JetCutting and rotating disc/nozzle technologies. Landbauforsch Völkenrode SH241:1–10
Zhang W, He X (2009) Encapsulation of living cells in small (∼ 100 μm) Alginate microcapsules by electrostatic spraying: a parametric study. J Biomed Eng. https://doi.org/10.1115/1.3153326
Gryshkov O, Pogozhykh D, Zernetsch H, Hofmann N, Mueller T, Glasmacher B (2014) Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl 36:77–83. https://doi.org/10.1016/j.msec.2013.11.048
Klokk TI, Melvik JE (2002) Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul 19:415–424. https://doi.org/10.1080/02652040210144234
Goosen MF, Al-Ghafri AS, Mardi OE, Al-Belushi MI, Al-Hajri HA, Mahmoud ES, Consolacion EC (1997) Electrostatic droplet generation for encapsulation of somatic tissue: assessment of high-voltage power supply. Biotechnol Prog 13:497–502
Lewińska D, Rosiński S, Weryński A (2004) Influence of process conditions during impulsed electrostatic droplet formation on size distribution of hydrogel beads. Artif Cells, Blood Substit Biotechnol 32:41–53
Xu Y, Hanna MA (2007) Electrosprayed bovine serum albumin-loaded tripolyphosphate cross-linked chitosan capsules: Synthesis and characterization. J Microencapsul 24:143–151. https://doi.org/10.1080/02652040601058434
Wilson JL, McDevitt TC (2013) Stem cell microencapsulation for phenotypic control, bioprocessing, and transplantation. Biotechnol Bioeng 110:667–682
Hallé J-P, Leblond FA, Pariseau J-F, Jutras P, Brabant M-J, Lepage Y (1994) Studies on small (<300 μm) microcapsules: II — parameters governing the production of alginate beads by high voltage electrostatic pulses. Cell Transplant 3:365–372. https://doi.org/10.1177/096368979400300503
Strand BL, Gåserød O, Kulseng B, Espevik T, Skjåk-Baek G (2002) Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul 19(5):615–630
Vossoughi A, Matthew HWT (2018) Encapsulation of mesenchymal stem cells in glycosaminoglycans-chitosan polyelectrolyte microcapsules using electrospraying technique: investigating capsule morphology and cell viability. Bioeng Transl Med 3:265–274. https://doi.org/10.1002/btm2.10111
Gryshkov O, Pogozhykh D, Hofmann N, Pogozhykh O, Mueller T, Glasmacher B (2014) Encapsulating non-human primate multipotent stromal cells in alginate via high voltage for cell-based therapies and cryopreservation. PLoS ONE 9:e107911. https://doi.org/10.1371/journal.pone.0107911
Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Anderson DG (2015) Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 14:643–651. https://doi.org/10.1038/nmat4290
Veiseh O, Vegas AJ (2019) Domesticating the foreign body response: recent advances and applications. Adv Drug Deliv Rev 144:148–161. https://doi.org/10.1016/j.addr.2019.08.010
An D, Chiu A, Flanders JA, Song W, Shou D, Lu YC, Grunnet LG, Winkel L, Ingvorsen C, Christophersen NS, Fels JJ, Sand FW, Ji Y, Qi L, Pardo Y, Luo D, Silberstein M, Fan J, Ma M (2018) Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci USA 115:E263–E272. https://doi.org/10.1073/pnas.1708806115
Lum Z-P, Krestow M, Tai IT, Vacek I, Sun AM (1992) Xenografts of rat islets into diabetic mice: an evaluation of new smaller capsules. Transplantation 53:1180–1183
Robitaille R, Pariseau J-F, Leblond FA, Lamoureux M, Lepage Y, Hallé J-P (1999) Studies on small alginate-poly-L-lysine microcapsules III Biocompatibility of smaller versus standard microcapsules. J Biomed Mater Res 44:116–120. https://doi.org/10.1002/(SICI)1097-4636(199901)44:1%3c116::AID-JBM13%3e3.0.CO;2-9
Tan J, Zhou G (2005) Chemokine receptors and transplantation. Cell Mol Immunol 2:343–349
Chung WY, Pollard CA, Kumar R, Drogemuller CJ, Naziruddin B, Stover C, Issa E, Isherwood J, Cooke J, Levy MF, Coates PTH, Garcea G, Dennison AR (2021) A comparison of the inflammatory response following autologous compared with allogenic islet cell transplantation. Ann Transl Med 9:98
Vaithilingam V, Evans MDM, Lewy DM, Bean PA, Bal S, Tuch BE (2017) Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci Rep 7:10059. https://doi.org/10.1038/s41598-017-10359-1
Kany S, Vollrath JT, Relja B (2019) Cytokines in inflammatory disease. Int J Mol Sci. https://doi.org/10.3390/ijms20236008
Mills C (2012) M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32:463–488. https://doi.org/10.1615/CritRevImmunol.v32.i6.10
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: EA, HB, GTK, FŞ and ÖKA. Acquisition of data: GDN, AAT, MK, EH and ÖKA. Analysis and interpretation of data: EA, GTK and ÖKA. Drafting or revising the manuscript: ÖKA, EA and GTK. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Human participants were treated in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Parathyroid tissues were obtained from the patients operated for subtotal parathyroidectomy for tertiary hyperparathyroidism.
Informed consent
Informed consent from the patients and the approval of the clinical studies ethical committee for the isolation of human cells (SBÜ Istanbul Training and Research Hospital, 11.07.2022, #217) were obtained. Rats were treated in accordance with the ethical guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals. Ethics committee approval was taken from Yeditepe University Local Ethics Committee for Animal Experiments (20.02.2021, 2021/01–5).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karabıyık Acar, Ö., Başoğlu, H., Keğin, M. et al. Microencapsulation of parathyroid cells via electric field and non-surgical transplantation approach. J Endocrinol Invest 46, 2257–2267 (2023). https://doi.org/10.1007/s40618-023-02075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02075-7