Abstract
Purpose
This study aimed to compare changes in the bone turnover markers (BTMs)—C-terminal telopeptide of type I collagen (CTX-I) and procollagen I N-terminal peptide (PINP)—with changes in the bone microarchitecture, assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT), during treatment of patients with thyroid dysfunction.
Methods
In women with newly diagnosed hypo- or hyperthyroidism, HR-pQCT variables, obtained from the tibia and the radius, were compared with BTMs. Data were collected at diagnosis and after at least 12 months of euthyroidism.
Results
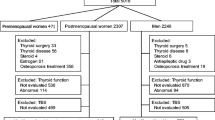
73 women completed the study (hypothyroidism, n = 27; hyperthyroidism, n = 46). Among hyperthyroid patients, correlations were found between changes in BTMs and HR-pQCT variables, primarily for cortical variables in the tibia, i.e. cortical thickness (CTX-I, p < 0.001; PINP, p < 0.001), and volumetric bone mass density (vBMD) (CTX-I, p < 0.001; PINP, p < 0.001). Moreover, correlations between BTMs and estimated bone strength were found. In the hypothyroid subgroup, no significant findings existed after adjustment. Following treatment, less decrease in tibial vBMD was seen among patients with increasing CTX-I compared to those with a decreasing CTX-I level (p = 0.009). Opposite findings applied to PINP, as patients with decreasing PINP showed an increase in tibial vBMD, in contrast to a decline in this parameter among patients with increasing PINP (p < 0.001).
Conclusion
Changes in CTX-I and PINP correlated with HR-pQCT variables during the treatment of women with thyroid dysfunction. To some extent, these BTMs reflected the restoration of bone microarchitecture. CTX-I seems to be the most sensitive BTM in treatment-naïve thyroid diseases, while PINP is more useful for monitoring during treatment.
Trial registration number
NCT02005250. Date: December 9, 2013.

Similar content being viewed by others
Availability of data and material
Data can be provided on request to the authors.
Code availability
Not applicable.
References
Bassett JH, Williams GR (2016) Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev 37(2):135–187
Williams GR, Bassett JHD (2018) Thyroid diseases and bone health. J Endocrinol Invest 41(1):99–109
Kenkre JS, Bassett J (2018) The bone remodelling cycle. Ann Clin Biochem 55(3):308–327
Gorka J, Taylor-Gjevre RM, Arnason T (2013) Metabolic and clinical consequences of hyperthyroidism on bone density. Int J Endocrinol 2013:638727
Vestergaard P, Mosekilde L (2003) Hyperthyroidism, bone mineral, and fracture risk–a meta-analysis. Thyroid 13(6):585–593
Vestergaard P, Mosekilde L (2002) Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid 12(5):411–419
Vestergaard P, Rejnmark L, Mosekilde L (2005) Influence of hyper- and hypothyroidism, and the effects of treatment with antithyroid drugs and levothyroxine on fracture risk. Calcif Tissue Int 77(3):139–144
Apostu D, Lucaciu O, Oltean-Dan D, Mureșan AD, Moisescu-Pop C, Maxim A et al (2020) The influence of thyroid pathology on osteoporosis and fracture risk: a review. Diagnostics 10(3):149
Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Brix TH, Hegedüs L (2014) Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res 29(9):2040–2050
Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR et al (2015) Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 313(20):2055–2065
Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH et al (2015) The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J Bone Miner Res 30(5):898–905
Eriksen EF, Mosekilde L, Melsen F (1986) Kinetics of trabecular bone resorption and formation in hypothyroidism: evidence for a positive balance per remodeling cycle. Bone 7(2):101–108
Delitala AP, Scuteri A, Doria C (2020) Thyroid hormone diseases and osteoporosis. J Clin Med 9(4):1034
Babu RP, Christy A, Hegde A, Manjrekar P, D’Souza V (2015) Do premenopausal hypothyroid women on levothyroxine therapy need bone status monitoring? Clin Med Insights Womens Health 8:1–6
Karimifar M, Esmaili F, Salari A, Kachuei A, Faragzadegan Z, Karimifar M (2014) Effects of Levothyroxine and thyroid stimulating hormone on bone loss in patients with primary hypothyroidism. J Res Pharm Pract 3(3):83–87
Langdahl BL, Loft AG, Eriksen EF, Mosekilde L, Charles P (1996) Bone mass, bone turnover and body composition in former hypothyroid patients receiving replacement therapy. Eur J Endocrinol 134(6):702–709
Moser E, Sikjaer T, Mosekilde L, Rejnmark L (2015) Bone indices in thyroidectomized patients on long-term substitution therapy with levothyroxine assessed by DXA and HR-pQCT. J Thyroid Res 2015:796871
Ock SY, Chung YS, Choi YJ (2016) Changes in bone mineral density and trabecular bone score in Graves’ disease patients after anti-thyroid therapy. Osteoporos Sarcopenia 2(3):175–179
Cheung AM, Adachi JD, Hanley DA, Kendler DL, Davison KS, Josse R et al (2013) High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the Canadian bone strength working group. Curr Osteoporos Rep 11(2):136–146
Sornay-Rendu E, Boutroy S, Duboeuf F, Chapurlat RD (2017) Bone microarchitecture assessed by HR-pQCT as predictor of fracture risk in postmenopausal women: The OFELY Study. J Bone Miner Res 32(6):1243–1251
Nicolaisen P, Obling ML, Winther KH, Hansen S, Hermann AP, Hegedüs L et al (2021) Consequences of hyperthyroidism and its treatment for bone microarchitecture assessed by high-resolution peripheral quantitative computed tomography. Thyroid 31(2):208–216
Obling ML, Nicolaisen P, Brix TH, Winther KH, Hansen S, Hegedüs L et al (2021) Restoration of euthyroidism in women with Hashimoto’s thyroiditis changes bone microarchitecture but not estimated bone strength. Endocrine 71(2):397–406
Williams C, Sapra A (2021) Osteoporosis Markers. StatPearls. StatPearls Publishing LLC, Treasure Island
Szulc P (2020) Biochemical bone turnover markers in hormonal disorders in adults: a narrative review. J Endocrinol Invest 43(10):1409–1427
Sabuncu T, Aksoy N, Arikan E, Ugur B, Tasan E, Hatemi H (2001) Early changes in parameters of bone and mineral metabolism during therapy for hyper- and hypothyroidism. Endocr Res 27(1–2):203–213
Konca Degertekin C, Turhan Iyidir O, Aktas Yılmaz B, Elbeg S, Pasaoglu OT, Pasaoglu H et al (2016) RANKL/Osteoprotegerin system and bone turnover in Hashimoto thyroiditis. Calcif Tissue Int 99(4):365–372
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS et al (2014) Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 24(12):1670–1751
Biondi B, Bartalena L, Cooper DS, Hegedüs L, Laurberg P, Kahaly GJ (2015) The 2015 European thyroid association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J 4(3):149–163
Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH (2018) 2018 European thyroid association guideline for the management of graves’ hyperthyroidism. Eur Thyroid J 7(4):167–186
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515
Laib A, Häuselmann HJ, Rüegsegger P (1998) In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6(5–6):329–337
Hansen S, Shanbhogue V, Folkestad L, Nielsen MM, Brixen K (2014) Bone microarchitecture and estimated strength in 499 adult Danish women and men: a cross-sectional, population-based high-resolution peripheral quantitative computed tomographic study on peak bone structure. Calcif Tissue Int 94(3):269–281
Mosekilde L, Christensen MS, Melsen F, Sørensen NS (1978) Effect of antithyroid treatment on calcium-phosphorus metabolism in hyperthyroidism. I: chemical quantities in serum and urine. Acta Endocrinol 87(4):743–750
Faber J, Jensen IW, Petersen L, Nygaard B, Hegedüs L, Siersbaek-Nielsen K (1998) Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol (Oxf) 48(3):285–290
Knudsen N, Faber J, Sierbaek-Nielsen A, Vadstrup S, Sørensen HA, Hegedüs L (1998) Thyroid hormone treatment aiming at reduced, but not suppressed, serum thyroid-stimulating hormone levels in nontoxic goitre: effects on bone metabolism amongst premenopausal women. J Intern Med 243(2):149–154
Balena R, Shih MS, Parfitt AM (1992) Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. J Bone Miner Res 7(12):1475–1482
Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM (2016) The periosteal bone surface is less mechano-responsive than the endocortical. Sci Rep 6:23480
Mikkola TM, Sipilä S, Rantanen T, Sievänen H, Suominen H, Kaprio J et al (2008) Genetic and environmental influence on structural strength of weight-bearing and non-weight-bearing bone: a twin study. J Bone Miner Res 23(4):492–498
Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA (2018) Diagnosis of endocrine disease: bone turnover markers: are they clinically useful? Eur J Endocrinol 178(1):R19-r31
Marques EA, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T et al (2016) Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: the AGES-Reykjavik longitudinal study. Osteoporos Int 27(12):3485–3494
Karga H, Papapetrou PD, Korakovouni A, Papandroulaki F, Polymeris A, Pampouras G (2004) Bone mineral density in hyperthyroidism. Clin Endocrinol (Oxf) 61(4):466–472
Eastell R, Szulc P (2017) Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol 5(11):908–923
Funding
This study received funding from “The Music Publishers Agnes and Knut Mørk’s Foundation”, from the “Danish Thyroid Federation”, and from research grants at Odense University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by The Regional Research Ethics Committee of Southern Denmark (S-2011–0018).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vinther, C.J., Poulsen, L.H., Nicolaisen, P. et al. Do bone turnover markers reflect changes in bone microarchitecture during treatment of patients with thyroid dysfunction?. J Endocrinol Invest 46, 345–358 (2023). https://doi.org/10.1007/s40618-022-01907-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01907-2