Abstract
Purpose
The hormonal thyroid changes related to obesity, even when in the euthyroid state, may contribute to the unfavorable cardio-metabolic profile of obese patients. In this retrospective study, we aim to investigate the biochemical thyroid changes and the association between serum TSH, FT4, FT3 and cardio-metabolic risk factors in euthyroid obese youths.
Methods
Four hundred ninety-one Caucasian euthyroid obese children and adolescents aged 9.93 ± 2.90 years were recruited. Each patient underwent clinical and auxological examination and laboratory workup including an OGTT and the measurement of thyroid function and lipid profile. Homeostasis model assessment of insulin resistance (HOMA-IR), triglyceride to high-density lipoprotein cholesterol ratio, total cholesterol to HDL ratio, atherogenic index of plasma, insulinogenic index, area under the glucose and insulin curves were calculated.
Results
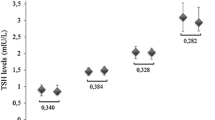
We found that TSH was positively correlated with BMI-SDS values and significantly associated with hypercholesterolemia and hyperinsulinemia; FT4 resulted negatively correlated with BMI-SDS; FT3 was positively correlated with BMI-SDS and the area under the curve of insulin and negatively correlated with HDL. FT3 and FT4 resulted significantly associated with severe obesity. In addition, children with high-normal TSH values showed higher triglyceride to high-density lipoprotein cholesterol ratio values than those with normal TSH levels.
Conclusions
Our data showed that thyroid hormones could influence obesity, lipid and glycemic parameters in euthyroid youths. These findings could carry implications regarding optimal TSH levels in obese children and confirm the importance of evaluating the thyroid function as possible adjunctive cardio-metabolic risk factor related to obesity.

Similar content being viewed by others
References
Corica D, Aversa T, Ruggeri RM, Cristani M, Alibrandi A, Pepe G et al (2019) Could AGE/RAGE-related oxidative homeostasis dysregulation enhance susceptibility to pathogenesis of cardio-metabolic complications in childhood obesity? Front Endocrinol (Lausanne) 10:426. https://doi.org/10.3389/fendo.2019.00426
Corica D, Aversa T, Valenzise M, Messina MF, Alibrandi A, De Luca F et al (2018) Does family history of obesity, cardiovascular, and metabolic diseases influence onset and severity of childhood obesity? Front Endocrinol (Lausanne) 9:187. https://doi.org/10.3389/fendo.2018.00187
Corica D, Oreto L, Pepe G, Calabro MP, Longobardo L, Morabito L et al (2020) Precocious preclinical cardiovascular sonographic markers in metabolically healthy and unhealthy childhood obesity. Front Endocrinol (Lausanne) 11:56. https://doi.org/10.3389/fendo.2020.00056
Tropeano A, Corica D, Li Pomi A, Pepe G, Morabito LA, Curatola SL et al (2021) The metabolic syndrome in pediatrics: do we have a reliable definition? A systematic review. Eur J Endocrinol 185(2):265–278. https://doi.org/10.1530/EJE-21-0238
Song RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA (2019) The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and meta-analysis. Front Immunol 10:2349. https://doi.org/10.3389/fimmu.2019.02349
Santini F, Marzullo P, Rotondi M, Ceccarini G, Pagano L, Ippolito S et al (2014) Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol 171(4):R137-152. https://doi.org/10.1530/EJE-14-0067
Garcia-Solis P, Garcia OP, Hernandez-Puga G, Sanchez-Tusie AA, Saenz-Luna CE, Hernandez-Montiel HL et al (2018) Thyroid hormones and obesity: a known but poorly understood relationship. Endokrynol Pol 69(3):292–303. https://doi.org/10.5603/EP.2018.0032
Chatzitomaris A, Hoermann R, Midgley JE, Hering S, Urban A, Dietrich B et al (2017) Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol (Lausanne) 8:163. https://doi.org/10.3389/fendo.2017.00163
Fontenelle LC, Feitosa MM, Severo JS, Freitas TE, Morais JB, Torres-Leal FL et al (2016) Thyroid function in human obesity: underlying mechanisms. Horm Metab Res 48(12):787–794. https://doi.org/10.1055/s-0042-121421
Longhi S, Radetti G (2013) Thyroid function and obesity. J Clin Res Pediatr Endocrinol 5(Suppl 1):40–44. https://doi.org/10.4274/jcrpe.856
Duntas LH, Biondi B (2013) The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid 23(6):646–653. https://doi.org/10.1089/thy.2011.0499
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85(9):660–667. https://doi.org/10.2471/blt.07.043497
Tanner JM (1955) Growth at adolescence. Blackwell Scientific Publications, Oxford, pp 212.
Camhi SM, Waring ME, Sisson SB, Hayman LL, Must A (2013) Physical activity and screen time in metabolically healthy obese phenotypes in adolescents and adults. J Obes 2013:984613. https://doi.org/10.1155/2013/984613
American Diabetes A (2017) 2. Classification and diagnosis of diabetes. Diabetes Care 40 (Suppl 1):S11–S24. https://doi.org/10.2337/dc17-S005
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Phillips DI, Clark PM, Hales CN, Osmond C (1994) Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 11(3):286–292. https://doi.org/10.1111/j.1464-5491.1994.tb00273.x
Haffner SM, Stern MP, Hazuda HP, Pugh JA, Patterson JK (1986) Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N Engl J Med 315(4):220–224. https://doi.org/10.1056/NEJM198607243150403
Dobiasova M, Frohlich J (2001) The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem 34(7):583–588. https://doi.org/10.1016/s0009-9120(01)00263-6
Barlow SE, Expert C (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4):S164-192. https://doi.org/10.1542/peds.2007-2329C
Valerio G, Licenziati MR, Iannuzzi A, Franzese A, Siani P, Riccardi G et al (2006) Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis 16(4):279–284. https://doi.org/10.1016/j.numecd.2005.12.007
Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J et al (2001) Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med 161(22):2685–2692. https://doi.org/10.1001/archinte.161.22.2685
Di Bonito P, Moio N, Scilla C, Cavuto L, Sibilio G, Sanguigno E et al (2012) Usefulness of the high triglyceride-to-HDL cholesterol ratio to identify cardiometabolic risk factors and preclinical signs of organ damage in outpatient children. Diabetes Care 35(1):158–162. https://doi.org/10.2337/dc11-1456
McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43(1):2–15. https://doi.org/10.1016/s0018-506x(02)00024-7
Witkowska-Sedek E, Kucharska A, Ruminska M, Pyrzak B (2017) Thyroid dysfunction in obese and overweight children. Endokrynol Pol 68(1):54–60. https://doi.org/10.5603/EP.2017.0007
Menendez C, Baldelli R, Camina JP, Escudero B, Peino R, Dieguez C et al (2003) TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol 176(1):7–12. https://doi.org/10.1677/joe.0.1760007
Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS (2002) Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 87(5):2352–2358. https://doi.org/10.1210/jcem.87.5.8472
Ferrante AW Jr (2007) Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 262(4):408–414. https://doi.org/10.1111/j.1365-2796.2007.01852.x
Antunes TT, Gagnon A, Bell A, Sorisky A (2005) Thyroid-stimulating hormone stimulates interleukin-6 release from 3T3-L1 adipocytes through a cAMP-protein kinase A pathway. Obes Res 13(12):2066–2071. https://doi.org/10.1038/oby.2005.256
Zhang YJ, Zhao W, Zhu MY, Tang SS, Zhang H (2013) Thyroid-stimulating hormone induces the secretion of tumor necrosis factor-alpha from 3T3-L1 adipocytes via a protein kinase A-dependent pathway. Exp Clin Endocrinol Diabetes 121(8):488–493. https://doi.org/10.1055/s-0033-1347266
Gagnon A, Langille ML, Chaker S, Antunes TT, Durand J, Sorisky A (2014) TSH signaling pathways that regulate MCP-1 in human differentiated adipocytes. Metabolism 63(6):812–821. https://doi.org/10.1016/j.metabol.2014.02.015
Felske D, Gagnon A, Sorisky A (2015) Interacting effects of TSH and insulin on human differentiated adipocytes. Horm Metab Res 47(9):681–685. https://doi.org/10.1055/s-0034-1395673
Frederiksen L, Brodbaek K, Fenger M, Jorgensen T, Borch-Johnsen K, Madsbad S et al (2002) Comment: studies of the Pro12Ala polymorphism of the PPAR-gamma gene in the Danish MONICA cohort: homozygosity of the Ala allele confers a decreased risk of the insulin resistance syndrome. J Clin Endocrinol Metab 87(8):3989–3992. https://doi.org/10.1210/jcem.87.8.8732
Cettour-Rose P, Theander-Carrillo C, Asensio C, Klein M, Visser TJ, Burger AG et al (2005) Hypothyroidism in rats decreases peripheral glucose utilisation, a defect partially corrected by central leptin infusion. Diabetologia 48(4):624–633. https://doi.org/10.1007/s00125-005-1696-4
Reinehr T, de Sousa G, Andler W (2006) Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab 91(8):3088–3091. https://doi.org/10.1210/jc.2006-0095
Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W (2008) Thyroid hormones and their relation to weight status. Horm Res 70(1):51–57. https://doi.org/10.1159/000129678
Stichel H, lallemand D, Gruters A (2000) Thyroid function and obesity in children and adolescents. Horm Res 54(1):14–19. https://doi.org/10.1159/000063431
Marras V, Casini MR, Pilia S, Carta D, Civolani P, Porcu M et al (2010) Thyroid function in obese children and adolescents. Horm Res Paediatr 73(3):193–197. https://doi.org/10.1159/000284361
Grandone A, Santoro N, Coppola F, Calabro P, Perrone L, Del Giudice EM (2010) Thyroid function derangement and childhood obesity: an Italian experience. BMC Endocr Disord 10:8. https://doi.org/10.1186/1472-6823-10-8
Toubro S, Sorensen TI, Ronn B, Christensen NJ, Astrup A (1996) Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab 81(7):2670–2674. https://doi.org/10.1210/jcem.81.7.8675595
Samuels MH, Kolobova I, Antosik M, Niederhausen M, Purnell JQ, Schuff KG (2017) Thyroid function variation in the normal range, energy expenditure, and body composition in L-T4-treated subjects. J Clin Endocrinol Metab 102(7):2533–2542. https://doi.org/10.1210/jc.2017-00224
Krause AJ, Cines B, Pogrebniak E, Sherafat-Kazemzadeh R, Demidowich AP, Galescu OA et al (2016) Associations between adiposity and indicators of thyroid status in children and adolescents. Pediatr Obes 11(6):551–558. https://doi.org/10.1111/ijpo.12112
Aeberli I, Jung A, Murer SB, Wildhaber J, Wildhaber-Brooks J, Knopfli BH et al (2010) During rapid weight loss in obese children, reductions in TSH predict improvements in insulin sensitivity independent of changes in body weight or fat. J Clin Endocrinol Metab 95(12):5412–5418. https://doi.org/10.1210/jc.2010-1169
Wang X, Gao X, Han Y, Zhang F, Lin Z, Wang H et al (2021) Causal association between serum thyrotropin and obesity: a bidirectional, mendelian randomization study. J Clin Endocrinol Metab 106(10):e4251–e4259. https://doi.org/10.1210/clinem/dgab183
Chin KY, Ima-Nirwana S, Mohamed IN, Aminuddin A, Johari MH, Ngah WZ (2014) The relationships between thyroid hormones and thyroid-stimulating hormone with lipid profile in euthyroid men. Int J Med Sci 11(4):349–355. https://doi.org/10.7150/ijms.7104
Xu C, Yang X, Liu W, Yuan H, Yu C, Gao L et al (2012) Thyroid stimulating hormone, independent of thyroid hormone, can elevate the serum total cholesterol level in patients with coronary heart disease: a cross-sectional design. Nutr Metab (Lond) 9(1):44. https://doi.org/10.1186/1743-7075-9-44
Asvold BO, Vatten LJ, Nilsen TI, Bjoro T (2007) The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol 156(2):181–186. https://doi.org/10.1530/eje.1.02333
Huang F, Wu L, Qiu Y, Bu K, Huang H, Li B (2019) The role of free triiodothyronine in high-density lipoprotein cholesterol metabolism. Med (Baltim) 98(36):e17016. https://doi.org/10.1097/MD.0000000000017016
Zhang J, Jiang R, Li L, Li P, Li X, Wang Z et al (2014) Serum thyrotropin is positively correlated with the metabolic syndrome components of obesity and dyslipidemia in chinese adolescents. Int J Endocrinol 2014:289503. https://doi.org/10.1155/2014/289503
Nader NS, Bahn RS, Johnson MD, Weaver AL, Singh R, Kumar S (2010) Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid 20(12):1333–1339. https://doi.org/10.1089/thy.2010.0180
Radhakishun NN, van Vliet M, von Rosenstiel IA, Weijer O, Beijnen JH, Brandjes DP et al (2013) Increasing thyroid-stimulating hormone is associated with impaired glucose metabolism in euthyroid obese children and adolescents. J Pediatr Endocrinol Metab 26(5–6):531–537. https://doi.org/10.1515/jpem-2012-0302
Witte T, Ittermann T, Thamm M, Riblet NB, Volzke H (2015) Association between serum thyroid-stimulating hormone levels and serum lipids in children and adolescents: a population-based study of german youth. J Clin Endocrinol Metab 100(5):2090–2097. https://doi.org/10.1210/jc.2014-4466
Tian L, Song Y, Xing M, Zhang W, Ning G, Li X et al (2010) A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology 52(4):1401–1409. https://doi.org/10.1002/hep.23800
Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA et al (2010) Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism 59(4):547–553. https://doi.org/10.1016/j.metabol.2009.08.018
Lin JZ, Martagon AJ, Hsueh WA, Baxter JD, Gustafsson JA, Webb P et al (2012) Thyroid hormone receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology 153(12):6136–6144. https://doi.org/10.1210/en.2011-2081
Tancevski I, Wehinger A, Demetz E, Eller P, Duwensee K, Huber J et al (2008) Reduced plasma high-density lipoprotein cholesterol in hyperthyroid mice coincides with decreased hepatic adenosine 5’-triphosphate-binding cassette transporter 1 expression. Endocrinology 149(7):3708–3712. https://doi.org/10.1210/en.2007-1387
Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J et al (2005) Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 102(29):10297–10302. https://doi.org/10.1073/pnas.0504379102
Prats-Puig A, Sitjar C, Ribot R, Calvo M, Clausell-Pomes N, Soler-Roca M et al (2012) Relative hypoadiponectinemia, insulin resistance, and increased visceral fat in euthyroid prepubertal girls with low-normal serum free thyroxine. Obes (Silver Spring) 20(7):1455–1461. https://doi.org/10.1038/oby.2011.206
Minami Y, Takaya R, Takitani K, Ishiro M, Okasora K, Niegawa T et al (2015) Association of thyroid hormones with obesity and metabolic syndrome in Japanese children. J Clin Biochem Nutr 57(2):121–128. https://doi.org/10.3164/jcbn.15-24
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
MW, AT and DC were involved in the conceptualization of the study; AA, AT and TA were involved in the design of the study; SLC, CC, ALP and GP were involved in the direct care of the patient, carrying out the data collection; AT and DC wrote the original draft; MW and TA were involved in the review of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Retrospective and anonymous analysis of data was notified to the Ethics Committee. The study was conducted according to the guidelines of the 1964 Declaration of Helsinki and approved by our Center’s Ethics Committee (Policlinico G. Martino, University of Messina), approval code number: 109/19, 17 February 2020.
Informed consent
All patients or parents/legal guardians on behalf of minors signed an informed consent for the use of their medical data for scientific research.
Research involving human participants and/or animals
The study research on humans was approved by our local ethical committee and it was conducted in line with the Declaration of Helsinky.
Consent to publish
Patients or parents/legal guardians on behalf of minors signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tropeano, A., Corica, D., Curatola, S. et al. The effect of obesity-related allostatic changes on cardio-metabolic risk in euthyroid children. J Endocrinol Invest 46, 285–295 (2023). https://doi.org/10.1007/s40618-022-01899-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01899-z