Abstract
Purpose
To retrospectively describe the association between thyroid hormones (TH) and platelet activation, as represented by mean platelet volume (MPV), in a cohort of patients hospitalized for COVID-19 with no known thyroid disease, and to correlate these data with the severity of COVID-19 and the occurrence of death/ARDS (Acute Respiratory Distress Syndrome).
Methods
103 patients with real-time polymerase chain reaction (RT-PCR) testing-confirmed COVID-19 and hospitalized were enrolled. Serum samples were collected from patients upon admission before starting any treatment. Chi-squared test was used to determine the association between euthyroid sick syndrome (ESS) and COVID-19 severity. Multivariate logistic regression was performed to evaluate the best independent predictors of COVID-19 deaths/ARDS.
Results
39/103 (37.9%) of patients were found to have ESS, and this condition was an independent predictor for the severity of COVID-19 (p = 0.003). Lower TSH and lower FT3/FT4 ratio correlated with higher MPV (p = 0,001 and p = 0.010), with an opposite trend with respect to what has been documented in non-COVID patients. Increasing MPV and lower FT3 significantly increased the risk, in COVID-19 patients, of an adverse outcome of death/ARDS.
Conclusion
Increased platelet activation, as represented by increased MPV, has already been reported to correlate with COVID-19 severity, possibly as a consequence of cytokine release. We demonstrated, in a cohort of 103 patients with COVID-19, that MPV is inversely correlated to TH levels, in particular in the case of ESS, where downregulation of TH axis may occur in case of systemic cytokine inflammation and more severe outcomes (death/ARDS). That ESS itself may directly cause platelet activation, as demonstrated by higher MPV in these patients, is an interesting hypothesis which deserves further investigation.
Similar content being viewed by others
Introduction
Thyroid dysfunction has been associated with a variety of abnormalities in coagulation. Although limited, the published studies suggest that patients with either hyperthyroidism and subclinical hypothyroidism have an increased thrombotic risk, whereas patients with overt hypothyroidism have a bleeding tendency, explained mainly by abnormalities of primary hemostasis resembling acquired von Willebrand disease [10] (Fig. 1). Thyroid hormones (TH) indeed have an effect on coagulation both via genomic pathways mediated by a nuclear TH receptor[11], and also via non-genomic mechanisms initiated at the receptor for L-thyroxine (T4) on platelet integrin αvβ3, or vitronectin receptor, with prothrombotic action [11, 12]. Increased levels of procoagulant factors, such as F VIII, F IX, fibrinogen, and Von Willebrand factor (vWF), have been found in hyperthyroid patients, with high levels of free Thyroxine (FT4) being associated with the risk of venous thrombosis, up to an odds ratio of 2.2 [13]. Also, increased platelet function has been demonstrated in patients with overt hyperthyroidism, compared to euthyroid ones, with time of platelet plug formation being shortened by therapy with thyroid-inhibitor thiamazole [14]. On the other hand, also subclinical hypothyroidism (SCH), defined as an increased thyroid-stimulating hormone (TSH) level with a normal FT4 level, has been associated with hypercoagulability [15,16,17]. In particular, in several cross-sectional studies, mean platelet volume (MPV) has a significant higher value in patients with SCH compared to euthyroid ones [18,19,20], although this has not been confirmed by larger-scale studies [21]. MPV indicates the mean platelet size and reflects the platelet production rate and activation [22]. Higher MPV has been correlated to a variety of pathological diseases [23], as a marker of severity, including the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Severe coronavirus disease 2019 (COVID-19 disease) has been associated with the increased production of larger, younger platelets [24], with higher MPV being correlated to a more severe course of the disease [25] and an increased risk of mortality [26]. In these patients, MPV may reflect the hyper-inflammatory state caused by the cytokine storm secondary to the infection, and responsible for many COVID-19-associated deaths [27]. Indeed, SARS-CoV-2 exhibits a marked respiratory tropism causing mainly interstitial pneumonia and acute respiratory distress syndrome (ARDS), but is also able to induce a severe systemic inflammation, leading to multi-organ dysfunction and even death in subjects with high-risk factors (i.e., old age, male gender, obesity, diabetes, and cardiovascular comorbidities) [1,2,3]. More severe manifestations of COVID-19 are associated with an excessive immune and inflammatory response, characterized by elevated serum levels of C-Reactive Protein (CRP), D-Dimer (D-D), Lactate Dehydrogenase (LDH), Neutrophil-to-Lymphocyte ratio (NLR), ferritin and several pro-inflammatory cytokines, the so-called “cytokine storm”[4, 5]. Apart from the typical lung involvement, patients with COVID-19 can experience several extra-pulmonary manifestations, including cardiovascular and neuromuscular disorders, renal failure, coagulopathies (thrombosis, disseminated intravascular coagulation, anemia, and thrombocytopenia), and also endocrinopathies. Preexisting endocrinopathies may worsen COVID-19 prognosis [6,7,8]. Thyroid gland involvement has been emerging as a quite common event in the course of COVID-19 [9], via both a direct and an indirect mechanism of SARS-CoV-2 infection that can render the thyroid dysfunctional [28]. The first one is mainly mediated by the direct expression on the thyroid gland surface of factors facilitating viral entry (ACE2), while the second, indirect one, is mainly represented by the potent cytokine storm elicited by the infection.
Thyroid dysfunction and hemostasis (readapted from [1])
Hence, given the well-known correlation between COVID-19 and, respectively, thyroid gland involvement on one side and increased platelet function on the other side, since the relationship between the latter two is still discussed, by means of a retrospective study on patients hospitalized for COVID-19 without known thyroid diseases, we hypothesized a possible association between TH levels and MPV values parallel to COVID-19 severity.
Methods
This retrospective study included 103 patients with real-time polymerase chain reaction (RT-PCR) testing-confirmed COVID-19 admitted to the Policlinico Umberto I hospital from April 2020 to March 2021. Demographic, epidemiological, clinical, laboratory, radiological, treatment, and outcome data were collected, and patients without a history of thyroid disease who had a thyroid function test at admission were enrolled. Patients underwent a routine laboratory screening at the entry of the Emergency Department including C-reactive protein (CRP), ferritin. Serum samples were collected from patients upon admission before starting any treatment. Patients with uncontrolled diabetes mellitus or hypertension or in treatment with diuretics, beta blockers, corticosteroids, anti-lipidemic agents which have effects on both platelets and thyroid function tests were excluded.
All patients underwent high-resolution chest computed tomography (CT) to evaluate the presence of interstitial pneumonia and its severity. Patients were classified as (1) no pneumonia if there was no radiological sign of pneumonia, (2) mild pneumoniae if there was only interstitial involvement without consolidation, (3) moderate pneumoniae if there was interstitial involvement with consolidation in less of 50% of lung parenchyma and (4) severe pneumoniae if there was interstitial involvement and consolidation in more than 50% of lung parenchyma.
Based on the severity of pulmonary impairment in Computer Tomography (CT) scan and respiratory failure in need of mechanical ventilation, patients were further divided into 4 groups according to the WHO guidelines updated in November 2021 [29]: patients with no CT scan alterations or hypoxia (Group 0-mild); patients with CT scan signs, suggestive of pneumonia with no oxygen supplementation (Group 1-moderate); patients with CT scan signs, suggestive of pneumonia plus oxygen supplementation (Group 2-severe) and patients with CT scan changes, suggestive of ARDS plus intensive care unit (ICU) admission (Group 3-critical). After the initial evaluation and management, patients were discharged in home isolation or were hospitalized in low, medium or sub-intensive/intensive care units according to medical needs. All patients were followed up to 60 days after the Emergency Department admission. Patients were further grouped following the occurrence within 2 months from admission of death/ARDS (Acute Respiratory Distress Syndrome) or not. Non-thyroidal illness syndrome (NTIS) and euthyroid sick syndrome (ESS) were defined as serum FT3 < 2.3 pg/ml with low or normal levels of TSH. Statistical analysis was performed using StatSoft, Inc. (2014) (data analysis software system), version 12. www.statsoft.com. Continuous variables are reported as mean and standard deviation or median and interquartile range depending on variable distribution. Squared linear regressions between continuous variables were always corrected for sex and age. Chi-squared test was used to determine the association between ESS and COVID-19 severity. Multivariate logistic regression was performed to evaluate the best independent predictors of COVID-19 deaths/ARDS.
Results
103 patients were included in the study, 57 females and 46 males. The mean age of the patients was 59.18 ± 17.18 years. The mean MPV was 9.04 ± 1.27 fL. The mean levels of TSH, free triiodothyronine (FT3) and FT4 were, respectively 1.69 ± 1.14 µU/ml (normal range 0.27–4.2); 2.50 ± 0.63 pg/ml (normal range 2–4.4); 1.43 ± 0.54 ng/dl (normal range 0.93–1.97).
14 patients showed no CT scan alterations or hypoxia (Group 0-mild); 7 patients were with CT scan signs suggestive of pneumonia with no oxygen supplementation (Group 1-moderate); 33 patients with CT scan signs suggestive of pneumonia plus oxygen supplementation (Group 2-severe) and 49 patients with CT scan changes suggestive of ARDS plus intensive care unit (ICU) admission (Group 3-critical). Patients were further grouped following the occurrence within 2 months from admission of death/ARDS—33 patients—or not—70 patients.
Linear regression analysis was performed to evaluate the relationships among TH levels and MPV, with results shown in Fig. 2. All the regressions were adjusted for age and sex. FT3 showed a trend of negative correlation with MPV (r = − 1704 p = 0.088), not statistically significant. FT4 was positively correlated with MPV (r = 2138, p = 0.037). Both TSH and FT3/FT4 ratio had a statistically significant inverse correlation with MPV (respectively, r = − 3258; p = 001 for TSH; r = − 2637, p = 010 for FT3/FT4).
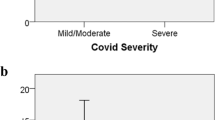
ESS was found in 39 patients (37.9%) 23 females, 16 males. Hence, based on this finding, patients were further divided into two groups according to serum FT3 values: the ESS group and the non-ESS group. Student’s T comparison revealed that ESS patients were, on average, older with respect to non-ESS patients (p < 0.001), having a higher MPV value (p = 0.027), a lower TSH, FT3 and FT3/FT4 ratio (p < 0.001) (Table 1). χ2 analysis revealed that ESS was more frequent with increasing severity of COVID-19 (p = 0.03) (Fig. 3).
Chi-squared test. Percentages of patients with (blue) or without (orange) euthyroid sick syndrome (ESS), in the different categories of severity. 0 mild course: no CT signs, 1 moderate course: CT signs + no O2 supplementation, 2 severe: CT signs + O2 supplementation, 3 critical: ARDS CT signs + Intensive Care Unit
One-way analysis of variance (Table 2) revealed that FT3 decreased with increasing severity of COVID-19 (p = 0.03) while both age and MPV increased with increasing severity (p = 0.03).
T test for independent samples was performed to evaluate differences between ARDS/Death patients and the control group (Table 3).
Multivariate logistic regression to evaluate independent predictors of COVID-19 severity (deaths/ARDS) showed that MPV (fL) was significantly positively related to the outcome, while FT3 (pg/ml) was negatively related (Table 4). In particular, when adjusted for comorbidities, increasing MPV and lower FT3 significantly increase the risk, in COVID-19 patients, of an adverse outcome of death/ARDS (p = 0.02 and p = 0.05, respectively).
Discussion
TH exerts effects on different levels of the hemostatic system (Fig. 1). It has been demonstrated that physiological concentrations of T4, but not T3, activate human platelets resulting in ATP release and aggregation [30]. Hyperthyroidism is associated with an increase in vWF activity levels [31] which may consecutively lead to increased cardiovascular risk [32]. On the other hand, also SCH has been found to be associated with hypercoagulability, reversible by 6 months of L-T4 treatment [17]. In particular, in several cross-sectional studies, MPV, a marker of platelet activation, has a statistically significant higher value in patients with SCH compared to euthyroid ones [18,19,20], suggesting a possible prothrombotic effect of elevated thyrotropin (TSH) [18]. Indeed, the association between SCH and coronary artery disease [33,34,35], as well as endothelial dysfunction [36, 37], is established, with an increased risk of cardiovascular events and mortality in those with higher TSH levels, particularly in those with a TSH concentration of 10 mIU/L or greater.
Platelets have a disk-shaped appearance in a quiescent state, but as they activate, they undergo a disk-to-sphere transformation with the development of pseudopodia, causing a subsequent increase in size (Fig. 1), which is mirrored by an increase in MPV [38]. Larger platelets are more metabolically and enzymatically active than smaller platelets, and have been found in several diseases, such as coronary arterial disease [39], insulin resistance [40], diabetes [41], dyslipidemia [42] and several inflammatory diseases [23]. COVID-19 represents the prototype of severe inflammatory disease, with the potent cytokine storm elicited by the infection being one of the main responsible of the COVID-19-associated deaths, along with pulmonary function impairment. SARS-CoV-2 is in fact a pleiotropic virus, known to exert its effects in many body organs other than the respiratory tract, such as the brain, heart, kidney [43], penis [44], and, not ultimately, thyroid [45]. In particular, whereas high TSH levels were reported only in up to 8% of patients with COVID-19, the most frequently reported abnormality, in 15 up to 56% of patients with COVID-19, is ESS [45,46,47] with patients typically presenting low or low-normal plasma T4, low plasma T3, increased plasma reverse T3 (rT3) concentrations in the absence of a rise in TSH. ESS has been found to occur in many critical illnesses [48], featuring different phases. In the acute one, usually changes in TH-binding predominantly occur, along with peripheral TH uptake, and alterations in the expression and activity of the type-1 and type-3 deiodinases. In particular, the former one, being the dominant enzyme driving the conversion of T4 to T3, is suppressed, while the second one, which contributes to the rise in rT3, is increased. Differently, in the prolonged phase of illness, there are alterations in the central regulation of the thyroid axis, with hypothalamic thyrotropin-releasing hormone (TRH) expression being suppressed, which explains reduced TSH secretion and therefore reduced TH release. ESS has been frequently reported in critically ill patients, where it represents more like an adaptive mechanism than a true dysfunction, in the sense that lowering the availability of the active T3 reduces energy expenditure to limit catabolism [47, 49]. As a matter of fact, the same mechanism appears to occur in healthy, fasting subjects [50].
Studies on COVID-19 patients found that FT3 and TSH were lower in the presence of more severe symptoms [28, 51, 52], while total T4 levels alone were not correlated with the gravity of the disease. This also occurred in our sample of patients, with ESS patients showing more severe signs of the disease with respect to non-ESS (Fig. 3) and more frequent occurrence of death/ARDS (Table 2, Fig. 5). Several studies have already correlated ESS to a poor outcome in patients with COVID-19 [49, 53, 54]. What it is interesting and relatively new in our study is that, in our sample of COVID-19 patients, other than with disease severity, lower TSH is correlated also with higher MPV. Our primary hypothesis is that, whether they already have an ESS, as demonstrated by low FT3 values found in 37.8% of patients, or they show a trend toward ESS, may partly explain such findings, as ESS usually occur in patients with acute, non-thyroidal, diseases, like sepsis, surgery or other types of physical stress, as a result of an adaptive mechanism to preserve energy. Hence, the higher the severity of COVID-19, the higher the degree of inflammation leading to hyperactive platelets as reflected by higher MPV, the higher the ESS, which in this case appears to be rather as a para-phenomenon of higher platelet activation. Indeed, the potent cytokine storm elicited by COVID-19 infection is able to over-activate the immune system with the subsequent massive production of inflammatory cytokines molecules [27, 55, 56]. This hyper-inflammatory state not only induces platelet activation [25, 55], but it is also responsible for severe damage to many vital organs [27]. The cytokines mainly involved in this process are IL1β, IL6, TNF-α, and IFN-γ, and, interestingly, some of them are also involved in immune-mediated thyroid diseases [56]. For instance, on the hypothalamus–pituitary–thyroid axis (HPT), they act by lowering TSH secretion, while, peripherally, they reduce the metabolism of TH via deiodinases by reducing the conversion of T4 to T3 and increasing the inactivation of both T4 and T3 [27], thereby possibly contributing to the ESS syndrome often found in COVID-19 patients. As a matter of fact, in patients with COVID-19 (and also in our sample), ESS occurs especially in those with higher clinical severity, who, therefore, may be in a state of higher systemic inflammation (as shown by higher MPV value) with respect to those with less severity. In particular, increasing MPV and lower FT3 significantly increase the risk of a poor outcome (p = 0.02 and p = 0.05, respectively). The hypothesis is that, in such patients, ESS may intervene precociously, acting as a sort of a “protective” condition to reduce catabolism in the setting of the hyper-inflammatory state driven by COVID-19, explaining the inverse relationship between TSH and MPV evidenced only in COVID-19 patients. On the other hand, a second, interesting hypothesis emerges from this study, that is, ESS itself may be the cause of abnormal platelet activation and higher MPV. Indeed, patients with nephrotic syndrome with ESS have been found to have abnormal platelet activation and increased platelet aggregation [57]. Pro-coagulative state associated with low T3 syndrome can be an important and previously undescribed mechanism that place patients with untreated ESS at high risk of thromboembolic complications. Indeed, it is well-known the association of ESS with greater disease severity, mortality, and unfavorable prognosis [58]. For instance, in patients with acute ischemic stroke, ESS was found to be an independent risk factor for hemorrhagic transformation [59]and poor functional outcome [60]. Moreover, ESS is independently associated with increased risks of all-cause mortality, cardiac mortality and major adverse cardiovascular events (MACE) in cardiovascular patients [61]. Whether supplementing thyroid hormones to raise FT3 levels into the normal range can help to ameliorate the outcomes of ESS patients is still unclear. However, considering a possible interaction of ESS with platelet activation and aggregation as a determinant of a worse prognosis in these patients, and to look at MPV as a simple and reliable marker of severity, can be an interesting field to explore. However, more clinical studies on patients with ESS, also due to other clinical conditions, and platelet activation are needed to establish this relationship.
Conclusion
This retrospective study aimed to assess the relationship between TH and platelet activation, as described by MPV, in a sample of hospitalized patients for COVID-19. 39 patients (37.9%) were found to have ESS, and, interestingly, their condition was a predictor of the severity of COVID-19, as shown by a combination of clinical and serological parameters. In our sample, lower TSH and lower FT3/FT4 ratio also correlated with higher MPV, with an opposite trend with respect to what has been documented in non-COVID patients. Also, ESS correlated with an adverse outcome (death or ARDS). Whether, in such patients, ESS may be a worse prognosis factor causing platelet activation, other than a functional adaptation able to lower catabolism in the presence of severe cytokine inflammation (and platelet activation) is an interesting hypothesis that, however, needs to be tested in a larger-scale study.
References
Huang C et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China The Lancet 395(10223):497–506
Guan W-J et al (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382(18):1708–1720
Watanabe, M., et al., Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism: clinical and experimental, 2020. 111: p. 154319–154319.
Gandini O et al (2021) Sex-disaggregated data confirm serum ferritin as an independent predictor of disease severity both in male and female COVID-19 patients. J Infect 82(3):414–451
Gandini O et al (2020) Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19. J Infect 81(6):979–997
Lubrano C et al (2020) Is Growth Hormone Insufficiency the Missing Link Between Obesity, Male Gender, Age, and COVID-19 Severity? Obesity (Silver Spring) 28(11):2038–2039
Masi, D., et al., Letter to the Editor: "Our Response to COVID-19 as Endocrinologists and Diabetologists". J Clin Endocrinol Metab, 2020. 105(7).
Puig-Domingo, M., et al., COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine, 2021. 72(2): p. 301–316.
Pal R, Banerjee M (2020) COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest 43(7):1027–1031
Franchini M et al (2009) Thyroid Dysfunction and Hemostasis: An Issue Still Unresolved. Semin Thromb Hemost 35(03):288–294
Davis PJ, Mousa SA, Schechter GP (2018) New Interfaces of Thyroid Hormone Actions With Blood Coagulation and Thrombosis. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 24(7):1014–1019
Davis PJ, Mousa SA, Lin H-Y (2020) Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol Rev 101(1):319–352
Debeij J et al (2014) High levels of procoagulant factors mediate the association between free thyroxine and the risk of venous thrombosis: the MEGA study. J Thromb Haemost 12(6):839–846
Homoncik M et al (2007) Altered Platelet Plug Formation in Hyperthyroidism and Hypothyroidism. J Clin Endocrinol Metab 92(8):3006–3012
Çakal, B., et al., Homocysteine and Fibrinogen Changes with L-thyroxine in Subclinical Hypothyroid Patients. jkms, 2007. 22(3): p. 431–435.
Akinci B et al (2007) Elevated Thrombin Activatable Fibrinolysis Inhibitor (TAFI) Antigen Levels in Overt and Subclinical Hypothyroid Patients Were Reduced by Levothyroxine Replacement. Endocr J 54(1):45–52
Gao F, Wang G, Xu J (2017) Alteration of Hemostatic Parameters in Patients with Different Levels of Subclinical Hypothyroidism and the Effect of L-thyroxine Treatment. Ann Clin Lab Sci 47(1):29–35
Kim JH et al (2012) The Mean Platelet Volume Is Positively Correlated with Serum Thyrotropin Concentrations in a Population of Healthy Subjects and Subjects with Unsuspected Subclinical Hypothyroidism. Thyroid 23(1):31–37
Yilmaz H et al (2011) Mean platelet volume in patients with subclinical hypothyroidism. Platelets 22(2):143–147
Erikci AA et al (2009) The effect of subclinical hypothyroidism on platelet parameters. Hematology 14(2):115–117
Ren X et al (2016) No associations exist between mean platelet volume or platelet distribution width and thyroid function in Chinese. Medicine (Baltimore) 95(40):e4573
Threatte GA (1993) Usefulness of the mean platelet volume. Clin Lab Med 13(4):937–950
Korniluk A et al (2019) Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediators Inflamm 2019:9213074
Daniels, S.A., H. Wei, and D.W. Denning, Platelet size as a predictor for severity and mortality in COVID-19 patients: a systematic review and meta-analysis. medRxiv, 2021.
Comer SP et al (2021) COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol 19(2):e3001109
Güçlü, E., et al., Effect of COVID-19 on platelet count and its indices. Rev Assoc Med Bras (1992), 2020. 66(8): p. 1122–1127.
Moore JB, June CH (2020) Cytokine release syndrome in severe COVID-19. Science 368(6490):473–474
Baldelli R et al (2021) Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest 44(12):2735–2739
Living guidance for clinical management of COVID-19: Living guidance, 23 November 2021 – World Health Organization (WHO) https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf.
Mousa SS et al (2010) Human platelet aggregation and degranulation is induced in vitro by L-thyroxine, but not by 3,5,3’-triiodo-L-thyronine or diiodothyropropionic acid (DITPA). Clin Appl Thromb Hemost 16(3):288–293
Erem C et al (2002) Blood coagulation and fibrinolysis in patients with hyperthyroidism. J Endocrinol Invest 25(4):345–350
Frossard M et al (2004) Platelet Function Predicts Myocardial Damage in Patients With Acute Myocardial Infarction. Circulation 110(11):1392–1397
Sun J et al (2017) Relationship between Subclinical Thyroid Dysfunction and the Risk of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. International Journal of Endocrinology 2017:8130796
Rodondi N et al (2010) Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304(12):1365–1374
Rodondi N et al (2006) Subclinical hypothyroidism and the risk of coronary heart disease: a meta-analysis. Am J Med 119(7):541–551
Niknam N et al (2016) Endothelial dysfunction in patients with subclinical hypothyroidism and the effects of treatment with levothyroxine. Adv Biomed Res 5:38–38
Cannarella, R., et al., Is There a Role for Levo-Thyroxine for the Treatment of Arterial Erectile Dysfunction? The Clinical Relevance of the Mean Platelet Volume. J Clin Med, 2020. 9(3).
Allen RD et al (1979) Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol 83(1):126–142
Sansanayudh N et al (2015) Prognostic effect of mean platelet volume in patients with coronary artery disease. Thromb Haemost 114(12):1299–1309
Varol E et al (2010) Mean platelet volume is associated with insulin resistance in non-obese, non-diabetic patients with coronary artery disease. J Cardiol 56(2):154–158
Papanas N et al (2004) Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 15(8):475–478
Pathansali R, Smith N, Bath P (2001) Altered megakaryocyte-platelet haemostatic axis in hypercholesterolaemia. Platelets 12(5):292–297
Libby P, Lüscher T (2020) COVID-19 is, in the end, an endothelial disease. Eur Heart J 41(32):3038–3044
Sansone A et al (2021) “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 9(4):1053–1059
Giovanella L et al (2021) Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clinical and translational imaging 9(3):233–240
Fliers E, Boelen A (2021) An update on non-thyroidal illness syndrome. J Endocrinol Invest 44(8):1597–1607
de Vries EM, Fliers E, Boelen A (2015) The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 225(3):R67-81
Van den Berghe G (2014) Non-Thyroidal Illness in the ICU: A Syndrome with Different Faces. Thyroid 24(10):1456–1465
Zou, R., et al., Euthyroid Sick Syndrome in Patients With COVID-19. Frontiers in Endocrinology, 2020. 11.
Boelen A, Wiersinga WM, Fliers E (2008) Fasting-Induced Changes in the Hypothalamus–Pituitary–Thyroid Axis. Thyroid 18(2):123–129
Gao W et al (2021) Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest 44(5):1031–1040
Chen M, Zhou W, Xu W (2021) Thyroid Function Analysis in 50 Patients with COVID-19: A Retrospective Study. Thyroid 31(1):8–11
Sparano C et al (2022) Euthyroid sick syndrome as an early surrogate marker of poor outcome in mild SARS-CoV-2 disease. J Endocrinol Invest 45(4):837–847
Schwarz Y et al (2021) Sick Euthyroid Syndrome on Presentation of Patients With COVID-19: A Potential Marker for Disease Severity. Endocr Pract 27(2):101–109
Coperchini F et al (2020) The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 53:25–32
Picchianti Diamanti, A., et al., Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Int J Mol Sci, 2020. 21(9).
Wu, J., et al., Low triiodothyronine syndrome is associated with platelet function in patients with nephrotic syndrome. Rev Assoc Med Bras (1992), 2019. 65(7): p. 988–992.
Bunevicius, A., Comments: "Low triiodothyronine syndrome is associated with platelet function in patients with nephrotic syndrome". Rev Assoc Med Bras (1992), 2019. 65(7): p. 993–994.
Huang GQ et al (2019) Low triiodothyronine syndrome is associated with hemorrhagic transformation in patients with acute ischaemic stroke. Aging (Albany NY) 11(16):6385–6397
Suda S et al (2016) Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J Neurol Sci 368:89–93
Wang B et al (2017) Non-thyroidal illness syndrome in patients with cardiovascular diseases: A systematic review and meta-analysis. Int J Cardiol 226:1–10
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional national research committee and with the 1964 Helsinki Declaration.
Informed consent
For this retrospective study no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colonnello, E., Criniti, A., Lorusso, E. et al. Thyroid hormones and platelet activation in COVID-19 patients. J Endocrinol Invest 46, 261–269 (2023). https://doi.org/10.1007/s40618-022-01896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01896-2