Abstract
Objective
To evaluate the causal effects of sleep traits (i.e., chronotype, insomnia, and sleep duration) on bioavailable testosterone (BT), sex hormone-binding globulin (SHBG), and total testosterone (TT) levels in women and men.
Methods
We performed Mendelian randomization (MR) using random-effect inverse-variance weighted (IVW) and 7 other MR analyses. Exposure data for sleep traits were obtained from the largest-to-date genome-wide association study (GWAS) from 339,926 to 1,331,010 individuals. Summary data for testosterone levels were obtained from GWAS based on the UK Biobank.
Results
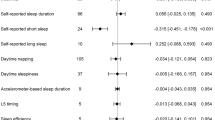
For women, our study supported that chronotype was associated with decreased BT (IVW: β = − 0.042, 95% CI − 0.060, − 0.023, p = 1.17E–05) and TT (IVW: − 0.053, 95% CI − 0.075, − 0.031, p = 2.30E–06). Besides, insomnia can significantly increase BT (IVW: β = 0.025, 95% CI 0.009, 0.041, p = 0.002). These findings were significant in most sensitivity analyses. For men, statistical significance was found between chronotype and BT (β = − 0.027, 95% CI − 0.048, − 0.005, p = 0.016), and insomnia and TT (β = − 0.028, 95% CI − 0.049, 0.007, p = 0.009) in IVW. However, the effect estimates were not broadly consistent with other sensitivity analyses. Our study did not find support for causal effects of sleep duration on testosterone levels in both women and men.
Conclusion
Our study reveals the sex differences in the effects of sleep traits on testosterone levels. A healthy sleep habit is vital for the maintenance of testosterone homeostasis in women. Further studies are warranted to investigate the associations between sleep traits and testosterone levels in men.


Similar content being viewed by others
References
Itani O, Jike M, Watanabe N, Kaneita Y (2017) Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 32:246–256
Andersen ML, Alvarenga TF, Mazaro-Costa R, Hachul HC, Tufik S (2011) The association of testosterone, sleep, and sexual function in men and women. Brain Res 1416:80–104. https://doi.org/10.1016/j.brainres.2011.07.060 (PMID: 21890115)
Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH et al (1989) Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab 69(3):533–539
Lim AJR, Huang Z, Chua SE, Kramer MS, Yong E-L (2016) Sleep duration, exercise, shift work and polycystic ovarian syndrome-related outcomes in a healthy population: a cross-sectional study. PLoS ONE 11(11):e0167048
Shifren JL, Davis SR (2017) Androgens in postmenopausal women: a review. Menopause 24(8):970–979
Liu PY, Takahashi PY, Yang RJ, Iranmanesh A, Veldhuis JD (2020) Age and time-of-day differences in the hypothalamo-pituitary-testicular, and adrenal, response to total overnight sleep deprivation. Sleep 43(7):zsaa008. https://doi.org/10.1093/sleep/zsaa008 (PMID: 31993665, PMCID: PMC7355405)
Smith I, Salazar I, RoyChoudhury A, St-Onge M-P (2019) Sleep restriction and testosterone concentrations in young healthy males: randomized controlled studies of acute and chronic short sleep. Sleep Health 5(6):580–586
Davies NM, Holmes MV, Davey SG (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601
Lawlor DA, Tilling K, Davey SG (2016) Triangulation in aetiological epidemiology. Int J Epidemiol 45(6):1866–1886
Zheng J, Haberland V, Baird D, Walker V, Haycock PC, Hurle MR et al (2020) Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet 52(10):1122–1131
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K et al (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726):203–209
Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S et al (2010) Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet 6(6):e1000993
Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764
Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN et al (2019) Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 10(1):343
Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR et al (2019) Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 51(3):394–403
Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H et al (2019) Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 10(1):1100
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A et al (2020) Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 26(2):252–258
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84(10):3666–3672
Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J (2021) The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol 50(5):1651–1659. https://doi.org/10.1093/ije/dyab084 (PMID: 33899104, PMCID: PMC8580269)
Lawlor DA (2016) Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol 45(3):908–915
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525
Slob EAW, Burgess S (2020) A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol 44(4):313–329
Verbanck M, Chen C-Y, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698
Morris CJ, Aeschbach D, Scheer FAJL (2012) Circadian system, sleep and endocrinology. Mol Cell Endocrinol 349:91
Suh S, Cho N, Zhang J (2018) Sex differences in insomnia: from epidemiology and etiology to intervention. Curr Psychiatry Rep 20(9):69
Zhang B, Wing Y-K (2006) Sex differences in insomnia: a meta-analysis. Sleep 29(1):85–93
Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R (2002) Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril 77(4):738–744
Hall JE, Sullivan JP, Richardson GS (2005) Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 90(4):2050–2055
Mong JA, Cusmano DM (2016) Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci 371(1688):20150110
Kische H, Ewert R, Fietze I, Gross S, Wallaschofski H, Völzke H et al (2016) Sex hormones and sleep in men and women from the general population: a cross-sectional observational study. J Clin Endocrinol Metab 101(11):3968–3977
Michels KA, Mendola P, Schliep KC, Yeung EH, Ye A, Dunietz GL et al (2020) The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol Int 37(2):260–271
Jankowski KS, Fajkowska M, Domaradzka E, Wytykowska A (2019) Chronotype, social jetlag and sleep loss in relation to sex steroids. Psychoneuroendocrinology 108:87–93
Peters EM, Arck PC, Paus R (2006) Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol 15(1):1–13. https://doi.org/10.1111/j.0906-6705.2005.00372.x (PMID: 16364026)
Kecklund G, Axelsson J (2016) Health consequences of shift work and insufficient sleep. BMJ (Clinical research ed) 355:i5210
Lucassen EA, Cizza G (2012) The hypothalamic-pituitary-adrenal axis, obesity, and chronic stress exposure: sleep and the HPA axis in obesity. Curr Obes Rep 1(4):208–215
McEwen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583(2–3):174–185
Hayes BL, Robinson T, Kar S, Ruth KS, Tsilidis KK, Frayling T et al (2022) Do sex hormones confound or mediate the effect of chronotype on breast and prostate cancer? A Mendelian randomization study. PLoS Genet 18(1):e1009887
Wang J, Fan X, Yang M, Song M, Wang K, Giovannucci E et al (2021) Sex-specific associations of circulating testosterone levels with all-cause and cause-specific mortality. Eur J Endocrinol 184(5):723–732
Hak AE, Westendorp IC, Pols HA, Hofman A, Witteman JC (2007) High-dose testosterone is associated with atherosclerosis in postmenopausal women. Maturitas 56(2):153–160
Wu IC, Lin XZ, Liu PF, Tsai WL, Shiesh SC (2010) Low serum testosterone and frailty in older men and women. Maturitas 67(4):348–352
Bachmann GA, Leiblum SR (1991) Sexuality in sexagenarian women. Maturitas 13(1):43–50
Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A et al (2006) Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 166(13):1380–1388
Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P (2001) Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab 86(3):1134–1139
Patel P, Shiff B, Kohn TP, Ramasamy R (2019) Impaired sleep is associated with low testosterone in US adult males: results from the National Health and Nutrition Examination Survey. World J Urol 37(7):1449–1453
Lord C, Sekerovic Z, Carrier J (2014) Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol (Paris) 62(5):302–310
Acknowledgements
We would like to thank the participants and investigators of the database we used in this study.
Besides, we would like to thank Mr. Xiaoyu Yu (Ph.D., Language Development Lab, Department of Linguistics, the University of Hong Kong) who provided professional language support to this manuscript.
Funding
This research was funded by Postdoctoral Research and Development Funds, West China Hospital, Sichuan University [2019HXBH087] & [2020HXBH016].
Author information
Authors and Affiliations
Contributions
The authors' contributions were as follows: CY, ZJ, and XJ conceived and designed the analysis; CY, ZJ, and XJ performed the analysis; CY and ZJ. wrote the manuscript; XJ reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
No ethics committee approval was conducted since this study did not involve animal or human experimentations or data containing personal information.
Informed Consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, C., Jian, Z. & Jin, X. Chronotype and insomnia may affect the testosterone levels with a sexual difference: a Mendelian randomization. J Endocrinol Invest 46, 123–132 (2023). https://doi.org/10.1007/s40618-022-01890-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01890-8