Abstract
Objective
Parathyroid scintigraphy is mandatory for the identification of hyperfunctioning parathyroid glands in hyperparathyroidism (HPT). The use of 99mTc-methoxy-isobutyl-isonitrile (MIBI) as radiopharmaceutical for parathyroid scintigraphy is considered the most valid and useful considering its uptake mechanism. Several MIBI-based radiopharmaceuticals are commercially available (i.e., MediMIBI, TechneMIBI, Stamicis). They seem to have similar physico-chemical characteristics and the choice between them is based on commercial criteria, even though some differences in qualitative scintigraphic results have been appreciated. Aims of the study were: first, to compare the scintigraphic quantitative data of MediMIBI, TechneMIBI, and Stamicis, particularly in the view of a personalized medicine approach; second, to investigate the potential effect of clinical-laboratory data on image quality using one of these radiopharmaceuticals.
Methods
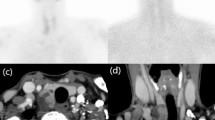
Patients with diagnosis of HPT, who underwent a parathyroid scintigraphy using one of the three MIBI-based radiopharmaceuticals between December 2018 and October 2020, have been retrospectively identified. Parameters derived from regions of interest (ROIs) drawn on three different sites were obtained: a reasonable parathyroid lesion detected, an area in the lateral neck considered as the background, and the hepatic dome as the site of MIBI physiological uptake. Laboratory and clinical data, such as serum calcium, PTH, vitamin D, and creatinine levels, as well as possible drug-mediated interferences were considered.
Results
Among 250 patients included, 83 (33.2%) had the parathyroid scintigraphy using MediMIBI, 84 (33.6%) using TechneMIBI and 83 (33.2%) using Stamicis. The ROIs on the parathyroid uptake at early images, on the background, and on the hepatic dome were statistically different among the three groups (p < 0.05). No significant differences were found in the remaining semi-quantitative parameters among the three groups, not even considering clinical-laboratory data.
Conclusions
Some differences in semi-quantitative parameters emerged among MIBI-based radiopharmaceuticals for parathyroid scintigraphy. This might justify the different qualitative scintigraphic results obtained using one or another of the cited radiopharmaceuticals.



Similar content being viewed by others
References
Mullan BP (2004) Nuclear medicine imaging of the parathyroid. Otolaryngol Clin North Am 37:909–939
Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY et al (2016) The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 151:959–968
Udelsman R, Lin Z, Donovan P (2011) The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg 253:585–591
Fraker DL, Harsono H, Lewis R (2009) Minimally invasive parathyroidectomy: benefits and requirements of localization, diagnosis, and intraoperative PTH monitoring. Long-term results world. J Surg 33:2256–2265
Bellantone R, Raffaelli M, de Crea C, Traini E, Lombardi CP (2011) Minimally-invasive parathyroid surgery. Acta Otorhinolaryngol Ital 31:207–215
Sukan A, Reyhan M, Aydin M, Yapar AF, Sert Y, Canpolat T et al (2008) Preoperative evaluation of hyperparathyroidism: the role of dual-phase parathyroid scintigraphy and ultrasound imaging. Ann Nucl Med 22:123–131
Noda S, Onoda N, Kashiwagi S, Kawajiri H, Takashima T, Ishikawa T et al (2014) Strategy of operative treatment of hyperparathyroidism using US scan and 99mTc-MIBI SPECT/CT. Endocr J 61:225–230
Vulpio C, Bossola M, De Gaetano A, Maresca G, Bruno I, Fadda G et al (2010) Usefulness of the combination of ultrasonography and 99mTc-sestamibi scintigraphy in the preoperative evaluation of uremic secondary hyperparathyroidism. Head Neck 32:1226–1235
Petranović Ovčariček P, Giovanella L, Carrió Gasset I, Hindié E, Huellner MW, Luster M et al (2021) The EANM practice guidelines for parathyroid imaging. Eur J Nucl Med Mol Imaging 48:2801–2822
Palestro CJ, Tomas MB, Tronco GG (2005) Radionuclide imaging of the parathyroid glands. Semin Nucl Med 35:266–276
ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology - International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Current Step 4 version, Parent Guideline 10/1994. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf
Annex 15 (EU) of Good Manufacturing Practice (GMP) guidelines: EDQM Guidelines: Guide for the elaboration of monographs on radiopharmaceutical preparations European Pharmacopoeia Edition (2018). https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-4/2015-10_annex15.pdf
Council of Europe Convention on the Elaboration of a European Pharmacopoeia. Detection and measurement of radioactivity. In: European Pharmacopoeia, 9th Edition 1, chapter 2.2.66 (2017)
Hindié E, Ugur O, Fuster D, O’Doherty M, Grassetto G, Ureña P et al (2009) 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging 36:1201–1216
Bruno I, Caldarella C (2017) Raccomandazioni procedurali AIMN: Scintigrafia Paratiroidea – Vrs. 03. www.aimn.it/documenti/lineeguida/13_LG%20procedurali%20sc%20%20paratiroidea%202017.pdf
Giordano A, Marozzi P, Meduri G, Ficola U, Calcagni ML, Vaccaro A et al (1999) Quantitative comparison of technetium-99m tetrofosmin and thallium-201 images of the thyroid and abnormal parathyroid glands. Eur J Nucl Med 26:907–911
Yang J, Wang H, Zhang J, Xu W, Weng W, Lv S et al (2021) Sestamibi single-positron emission computed tomography/diagnostic-quality computed tomography for the localization of abnormal parathyroid glands in patients with primary hyperparathyroidism: What clinicopathologic factors affect its accuracy? J Endocrinol Invest 44:1649–1658
Fuster D, Torregrosa J-V, Domenech B, Solà O, Martín G, Casellas J et al (2009) Dual-phase 99mTc-MIBI scintigraphy to assess calcimimetic effect in patients on haemodialysis with secondary hyperparathyroidism. Nucl Med Commun 30:890–894
Friedman K, Somervell H, Patel P, Melton GB, Garrett-Mayer E, Dackiw APB et al (2004) Effect of calcium channel blockers on the sensitivity of preoperative 99mTc-MIBI SPECT for hyperparathyroidism. Surgery 136:1199–1204
Karam M, Dansereau RN, Dolce CJ, Feustel PJ, Robinson LW (2005) Increasing the radiochemical purity of 99mTc sestamibi commercial preparations results in improved sensitivity of dual-phase planar parathyroid scintigraphy. Nucl Med Commun 26:1093–1098
Khorasani N, Mohammadi A (2014) Effective factors on the sensitivity of preoperative sestamibi scanning for primary hyperparathyroidism. Int J Clin Exp Med 7:2639–2644
Hughes DT, Sorensen MJ, Miller BS, Cohen MS, Gauger PG (2014) The biochemical severity of primary hyperparathyroidism correlates with the localization accuracy of sestamibi and surgeon-performed ultrasound. J Am Coll Surg 219:1010–1019
Dugonjić S, Šišić M, Radulović M, Ajdinović B (2017) Positive 99mTc-MIBI and the subtraction parathyroid scan are related to intact parathyroid hormone but not to total plasma calcium in primary hyperparathyroidism. Hell J Nucl Med 20:46–50
Maccora D, Rizzo V, Fortini D, Mariani M, Giraldi L, Giordano A et al (2019) Parathyroid scintigraphy in primary hyperparathyroidism: comparison between double-phase and subtraction techniques and possible affecting factors. J Endocrinol Invest 42:889–895
Bandeira FA, Oliveira RI, Griz LH, Caldas G, Bandeira C (2008) Differences in accuracy of 99mTc-sestamibi scanning between severe and mild forms of primary hyperparathyroidism. J Nucl Med Technol 36:30–35
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest to disclose.
Ethical approval
This study has been approved by the appropriate ethics committee, and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maccora, D., Fortini, D., Moroni, R. et al. Comparison between MIBI-based radiopharmaceuticals for parathyroid scintigraphy: quantitative evaluation and correlation with clinical-laboratory parameters. J Endocrinol Invest 45, 2139–2147 (2022). https://doi.org/10.1007/s40618-022-01847-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01847-x