Abstract
Purpose
Acromegaly caused by growth hormone cell adenoma is commonly associated with abnormal glucolipid metabolism, which may result from changes in adipocytokine secretion. This study aims to investigate serum adipokine levels, including pro-neurotensin (PNT), furin, and zinc alpha-2-glycoprotein (ZAG), in acromegalic patients and the correlation between the levels of these three adipokines and GH levels and glucolipid metabolism indices.
Methods
Sixty-eight acromegalic patients and 121 controls were included, and their clinical data were recorded from electronic medical record system. Serum PNT, furin and ZAG levels were measured by ELISA.
Results
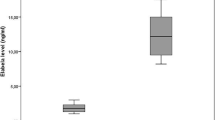
Serum PNT levels in acromegalic patients were significantly higher than controls (66.60 ± 12.36 vs. 46.68 ± 20.54 pg/ml, P < 0.001), and acromegaly was an independent influencing factor of PNT levels (P < 0.001). Moreover, subjects with the highest tertile of PNT levels had a close correlation with acromegaly (OR = 22.200, 95% CI 7.156 ~ 68.875, P < 0.001), even in Model 1 adjusted for gender and age and Model 2 adjusted for gender, age and BMI. Additionally, serum PNT levels were positively correlated with BMI (r = 0.220, P = 0.002) and triglycerides (TGs, r = 0.295, P < 0.001), and TGs were an independent influencing factor of serum PNT levels in acromegalic subjects (P < 0.001). Furthermore, serum PNT levels in obese acromegalic patients were significantly higher than those with normal BMI (P < 0.05). However, serum furin levels were lower in acromegalic patients than controls (0.184 ± 0.036 vs. 0.204 ± 0.061 ng/ml, P < 0.001).
Conclusion
This study is the first to demonstrate that acromegalic patients have increased serum PNT levels. Moreover, serum PNT plays a potential role in abnormal lipid metabolism of acromegalic patients.



Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA (2017) Acromegaly: clinical features at diagnosis. Pituitary 20(1):22–32. https://doi.org/10.1007/s11102-016-0772-8
Olarescu NC, Bollerslev J (2016) The impact of adipose tissue on insulin resistance in acromegaly. Trends Endocrinol Metab 27(4):226–237. https://doi.org/10.1016/j.tem.2016.02.005
Berryman DE, List EO (2017) Growth hormone’s effect on adipose tissue: quality versus quantity. Int J Mol Sci 18(8):1621. https://doi.org/10.3390/ijms18081621
Mercado M, Ramírez-Rentería C (2018) Metabolic complications of acromegaly. Front Hormon Res 49:20–28. https://doi.org/10.1159/000486001
Cozzo AJ, Fuller AM, Makowski L (2017) Contribution of adipose tissue to development of cancer. Compr Physiol 8(1):237–282. https://doi.org/10.1002/cphy.c170008
Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M (2020) Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci 21(10):3570. https://doi.org/10.3390/ijms21103570
Schroeder LE, Leinninger GM (1864) (2018) Role of central neurotensin in regulating feeding: implications for the development and treatment of body weight disorders Biochimica et biophysica acta. Mol Basis Dis 3:900–916. https://doi.org/10.1016/j.bbadis.2017.12.036
Brown DR, Miller RJ (1982) Neurotensin. Br Med Bull 38(3):239–245. https://doi.org/10.1093/oxfordjournals.bmb.a071767
Rostène WH, Alexander MJ (1997) Neurotensin and neuroendocrine regulation. Front Neuroendocrinol 18(2):115–173. https://doi.org/10.1006/frne.1996.0146
Fawad A, Bergmann A, Struck J, Nilsson PM, Orho-Melander M, Melander O (2018) Proneurotensin predicts cardiovascular disease in an elderly population. J Clin Endocrinol Metab 103(5):1940–1947. https://doi.org/10.1210/jc.2017-02424
Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Evers BM (2016) An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 533(7603):411–415. https://doi.org/10.1038/nature17662
Nicoli CD, Wettersten N, Judd SE, Howard G, Howard VJ, Struck J, Cushman M (2020) Pro-neurotensin/neuromedin N and risk of ischemic stroke: The REasons for GEOGRAPHIC And Racial Differences in Stroke (REGARDS) study. Vasc Med (London, England) 25(6):534–540. https://doi.org/10.1177/1358863X20957406
Cimini FA, Barchetta I, Bertoccini L, Ceccarelli V, Baroni MG, Melander O, Cavallo MG (2022) High pro-neurotensin levels in individuals with type 1 diabetes associate with the development of cardiovascular risk factors at follow-up. Acta Diabetol 59(1):49–56. https://doi.org/10.1007/s00592-021-01783-x
Villar B, Bertran L, Aguilar C, Binetti J, Martínez S, Sabench F, Real M, Riesco D, París M, Del Castillo D, Richart C, Auguet T (2021) Circulating levels of pro-neurotensin and its relationship with nonalcoholic steatohepatitis and hepatic lipid metabolism. Metabolites 11(6):373. https://doi.org/10.3390/metabo11060373
Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, Engström G, Kilger U, Nilsson P, Bergmann A, Orho-Melander M (2012) Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 308(14):1469–1475. https://doi.org/10.1001/jama.2012.12998
Scamuffa N, Calvo F, Chrétien M, Seidah NG, Khatib AM (2006) Proprotein convertases: lessons from knockouts. FASEB J 20(12):1954–1963. https://doi.org/10.1096/fj.05-5491rev
Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, Essalmani R, Prat A (2008) The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol 40(6–7):1111–1125. https://doi.org/10.1016/j.biocel.2008.01.030
Uhlén M, Björling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergström K, Brumer H, Cerjan D, Ekström M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, Pontén F (2005) A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4(12):1920–1932. https://doi.org/10.1074/mcp.M500279-MCP200
Bravo DA, Gleason JB, Sanchez RI, Roth RA, Fuller RS (1994) Accurate and efficient cleavage of the human insulin proreceptor by the human proprotein-processing protease furin. Characterization and kinetic parameters using the purified, secreted soluble protease expressed by a recombinant baculovirus. J Biol Chem 269(41):25830–25837
Yoshimasa Y, Seino S, Whittaker J, Kakehi T, Kosaki A, Kuzuya H, Imura H, Bell GI, Steiner DF (1988) Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science(New York, N Y) 240(4853):784–787. https://doi.org/10.1126/science.3283938
Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, Ruskoaho H, Melander O (2018) Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 284(4):377–387. https://doi.org/10.1111/joim.12783
Liu ZW, Ma Q, Liu J, Li JW, Chen YD (2021) The association between plasma furin and cardiovascular events after acute myocardial infarction. BMC Cardiovasc Disord 21(1):468. https://doi.org/10.1186/s12872-021-02029-y
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P (2004) Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 101(8):2500–2505. https://doi.org/10.1073/pnas.0308647100
Pearsey HM, Henson J, Sargeant JA, Davies MJ, Khunti K, Suzuki T, Bowden-Davies KA, Cuthbertson DJ, Yates TE (2020) Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance: a systematic review and meta-analysis. Rev Endocr Metabol Disord 21(4):569–575. https://doi.org/10.1007/s11154-020-09553-w
Severo JS, Morais J, Beserra JB, Dos Santos LR, de Sousa Melo SR, de Sousa GS, de Matos Neto EM, Henriquesdo Nascimento Marreiro, D GS (2020) Role of zinc in zinc-α2-glycoprotein metabolism in obesity: a review of literature. Biol Trace Elem Res 193(1):81–88. https://doi.org/10.1007/s12011-019-01702-w
Kawamata T, Inui A, Hosoda H, Kangawa K, Hori T (2007) Perioperative plasma active and total ghrelin levels are reduced in acromegaly when compared with in nonfunctioning pituitary tumours even after normalization of serum GH. Clini Endocrinol 67(1):140–144. https://doi.org/10.1111/j.1365-2265.2007.02851.x
Sucunza N, Barahona MJ, Resmini E, Fernández-Real JM, Ricart W, Farrerons J, Rodríguez Espinosa J, Marin AM, Puig T, Webb SM (2009) A link between bone mineral density and serum adiponectin and visfatin levels in acromegaly. J Clin Endocrinol Metab 94(10):3889–3896. https://doi.org/10.1210/jc.2009-0474
Ke X, Duan L, Gong F, Zhang Y, Deng K, Yao Y, Wang L, Pan H, Zhu H (2020) Serum levels of asprosin, a novel adipokine, are significantly lowered in patients with acromegaly. Int J Endocrinol 2020:8855996. https://doi.org/10.1155/2020/8855996
Banskota S, Adamson DC (2021) Pituitary adenomas: from diagnosis to therapeutics. Biomedicines 9(5):494. https://doi.org/10.3390/biomedicines9050494
Zhu H, Xu Y, Gong F, Shan G, Yang H, Xu K, Zhang D, Cheng X, Zhang Z, Chen S, Wang L, Pan H (2017) Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS ONE 12(10):e0185561. https://doi.org/10.1371/journal.pone.0185561
Giorgi RR, Chile T, Bello AR, Reyes R, Fortes MA, Machado MC, Cescato VA, Musolino NR, Bronstein MD, Giannella-Neto D, Corrêa-Giannella ML (2008) Expression of neurotensin and its receptors in pituitary adenomas. J Neuroendocrinol 20(9):1052–1057. https://doi.org/10.1111/j.1365-2826.2008.01761.x
Schimpff RM, Bozzola M, Lhiaubet AM, Spadaro B, Tettoni K, Rostène W (1994) Effects of growth hormone administration on neurotensin release in children with growth delay. Horm Res 42(3):95–99. https://doi.org/10.1159/000184155
Vijayan E, McCann SM (1980) Effects of substance P and neurotensin on growth hormone and thyrotropin release in vivo and in vitro. Life Sci 26(4):321–327. https://doi.org/10.1016/0024-3205(80)90344-6
Li L, Weiss HL, Li J, Chen Z, Donato L, Evers BM (2020) High plasma levels of pro-NT are associated with increased colon cancer risk. Endocr-Related Cancer 27(11):641–646. https://doi.org/10.1530/ERC-20-0310
Barchetta I, Cimini FA, Capoccia D, Bertoccini L, Ceccarelli V, Chiappetta C, Leonetti F, Di Cristofano C, Silecchia G, Orho-Melander M, Melander O, Cavallo MG (2018) Neurotensin is a lipid-induced gastrointestinal peptide associated with visceral adipose tissue inflammation in obesity. Nutrients 10(4):526. https://doi.org/10.3390/nu10040526
Barchetta I, Cimini FA, Leonetti F, Capoccia D, Di Cristofano C, Silecchia G, Orho-Melander M, Melander O, Cavallo MG (2018) Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 diabetes. J Clin Endocrinol Metab 103(6):2253–2260. https://doi.org/10.1210/jc.2017-02751
Wei Z, Lei X, Seldin MM, Wong GW (2012) Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 287(43):35804–35814. https://doi.org/10.1074/jbc.M112.365965
Guo X, Gao L, Shi X, Li H, Wang Q, Wang Z, Chen W, Xing B (2018) Pre- and postoperative body composition and metabolic characteristics in patients with acromegaly: a prospective study. Int J Endocrinol 2018:4125013. https://doi.org/10.1155/2018/4125013
Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, Sarkar P, Rendon DA, Gaber MW, LeMaire SA, Coselli JS, Milewicz DM, Sutton VR, Butte NF, Moore DD, Chopra AR (2016) Asprosin, a fasting-induced glucogenic protein hormone. Cell 165(3):566–579. https://doi.org/10.1016/j.cell.2016.02.063
Tada T, Ohkubo I, Niwa M, Sasaki M, Tateyama H, Eimoto T (1991) Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. J of Histochem Cytochem 39(9):1221–1226. https://doi.org/10.1177/39.9.1918940
Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V, Guigné C, Carpéné C, Valet P, Gilfillan S, Bahram S (2007) Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 581(3):394–400. https://doi.org/10.1016/j.febslet.2006.12.047
Wei X, Liu X, Tan C, Mo L, Wang H, Peng X, Deng F, Chen L (2019) Expression and function of zinc-α2-glycoprotein. Neurosci Bull 35(3):540–550. https://doi.org/10.1007/s12264-018-00332-x
Balaz M, Ukropcova B, Kurdiova T, Gajdosechova L, Vlcek M, Janakova Z, Fedeles J, Pura M, Gasperikova D, Smith SR, Tkacova R, Klimes I, Payer J, Wolfrum C, Ukropec J (2015) Adipokine zinc-α2-glycoprotein regulated by growth hormone and linked to insulin sensitivity. Obesity (Silver Spring, Md) 23(2):322–328. https://doi.org/10.1002/oby.20856
Rydén M, Agustsson T, Andersson J, Bolinder J, Toft E, Arner P (2012) Adipose zinc-α2-glycoprotein is a catabolic marker in cancer and noncancerous states. J Intern Med 271(4):414–420. https://doi.org/10.1111/j.1365-2796.2011.02441.x
Fain JN, Tagele BM, Cheema P, Madan AK, Tichansky DS (2010) Release of 12 adipokines by adipose tissue, nonfat cells, and fat cells from obese women. Obesity (Silver Spring, Md) 18(5):890–896. https://doi.org/10.1038/oby.2009.335
Acknowledgements
We thank the staff of Clinical Biobank, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences for their hard work in sample storage.
Funding
This work was supported by a grant from the CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1–003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Ethics Committee of the Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (ethics code: JS-1663). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Research involving human participants and/or animals
The study was approved by our local ethical committee and informed consent was obtained.
Informed consent
All subjects provided with fully informed consent before the study and consent was obtained form all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ke, X., Duan, L., Gong, F. et al. A study on serum pro-neurotensin (PNT), furin, and zinc alpha-2-glycoprotein (ZAG) levels in patients with acromegaly. J Endocrinol Invest 45, 1945–1954 (2022). https://doi.org/10.1007/s40618-022-01827-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01827-1