Abstract
Purpose
No single reliable biomarker is available for nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). Vasostatin-1 (VS-1), the N-terminal fragment of chromogranin A (CgA), seems to be a more accurate biomarker compared to its precursor. Primary aim was to investigate the ability of VS-1, compared to total-CgA, to assess the effectiveness of surgical resection performed for NF-PanNETs. Secondary aim was to evaluate two additional CgA-derived fragments, pancreastatin (PST) and vasostatin-2 (VS-2), as possible biomarkers for NF-PanNETs.
Methods
Consecutive patients who underwent surgery for NF-PanNETs at San Raffaele Scientific Institute were included (n = 35). Plasma levels of CgA and CgA-derived fragments were measured by Enzyme-Linked ImmunoSorbent Assay (ELISA), preoperatively and postoperatively.
Results
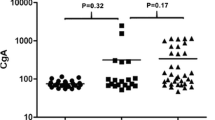
Preoperative VS-1 was significantly higher compared to VS-1 measured on postoperative day 5 (POD5) (pre: 0.338 nM versus POD5: 0.147 nM, P < 0.001), whereas total-CgA significantly increased after surgery (pre: 1.123 nM versus POD5: 1.949 nM, P = 0.006). Overall, 24 patients showed ≥ 1 feature of tumor aggressiveness (T3-T4, nodal/distant metastases, Ki67 > 5%, microvascular/perineural invasion, necrosis). The median percentage decrease in VS-1 plasma levels was 63% (IQR 28–88%) among patients with aggressive tumors, compared to 13% (IQR 0–57%) in the remaining population (P = 0.033). No significant differences in terms of PST (P = 0.870) and VS-2 (P = 0.909) were observed between preoperative and postoperative time.
Conclusion
VS-1 provides an early assessment of surgical efficacy in patients who undergo resection for NF-PanNETs, especially in those with aggressive neoplasms. Total-CgA, PST and VS-2 have no clinical utility in this setting.


Similar content being viewed by others
Availability of data and material
All the data generated or analyzed during this study are included in the manuscript or in the supplementary material.
Code availability
Not applicable.
References
Dasari A, Shen C, Halperin D et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342. https://doi.org/10.1001/jamaoncol.2017.0589
Vagefi PA, Razo O, Deshpande V et al (2007) Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg 142:347–354. https://doi.org/10.1001/archsurg.142.4.347
Falconi M, Eriksson B, Kaltsas G et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103:153–371. https://doi.org/10.1159/000443171
Genç CG, Falconi M, Partelli S et al (2018) Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol 25:2467–2474. https://doi.org/10.1245/s10434-018-6518-2
Hashim YM, Trinkaus KM, Linehan DC et al (2014) Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg 259:197–203. https://doi.org/10.1097/SLA.0000000000000348
Partelli S, Javed AA, Andreasi V et al (2018) The number of positive nodes accurately predicts recurrence after pancreaticoduodenectomy for nonfunctioning neuroendocrine neoplasms. Eur J Surg Oncol 44:778–783. https://doi.org/10.1016/j.ejso.2018.03.005
Zaidi MY, Lopez-Aguiar AG, Switchenko JM et al (2019) A novel validated recurrence risk score to guide a pragmatic surveillance strategy after resection of pancreatic neuroendocrine tumors: an international study of 1006 patients. Ann Surg 270:422–433. https://doi.org/10.1097/SLA.0000000000003461
Howe JR, Merchant NB, Conrad C et al (2020) The North American neuroendocrine tumor society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 49:1–33. https://doi.org/10.1097/MPA.0000000000001454
Jilesen APJ, Busch ORC, van Gulik TM et al (2014) Standard pre- and postoperative determination of chromogranin a in resectable non-functioning pancreatic neuroendocrine tumors–diagnostic accuracy: NF-pNET and low tumor burden. Dig Surg 31:407–414. https://doi.org/10.1159/000370007
Partelli S, Andreasi V, Muffatti F et al (2020) Circulating neuroendocrine gene transcripts (NETest): a postoperative strategy for early identification of the efficacy of radical surgery for pancreatic neuroendocrine tumors. Ann Surg Oncol 27:3928–3936. https://doi.org/10.1245/s10434-020-08425-6
Marotta V, Zatelli MC, Sciammarella C et al (2018) Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer 25:R11–R29. https://doi.org/10.1530/ERC-17-0269
Oberg K (2011) Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 18(Suppl 1):S17-25. https://doi.org/10.1530/ERC-10-0280
Andreasi V, Partelli S, Muffatti F et al (2021) Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver 53:171–182. https://doi.org/10.1016/j.dld.2020.08.031
Giusti M, Sidoti M, Augeri C et al (2004) Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol 150:299–303. https://doi.org/10.1530/eje.0.1500299
Pregun I, Herszenyi L, Juhasz M et al (2011) Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 84:22–28. https://doi.org/10.1159/000321535
Dam G, Grønbæk H, Sorbye H et al (2020) Prospective study of chromogranin a as a predictor of progression in patients with pancreatic, small-intestinal, and unknown primary neuroendocrine tumors. Neuroendocrinology 110:217–224. https://doi.org/10.1159/000503833
Brehm Hoej L, Parkner T, Soendersoe Knudsen C, Grønbaek H (2014) A comparison of three chromogranin A assays in patients with neuroendocrine tumours. J Gastrointestin Liver Dis 23:419–424. https://doi.org/10.15403/jgld.2014.1121.234.3ca
Baekdal J, Krogh J, Klose M et al (2020) Limited diagnostic utility of chromogranin a measurements in workup of neuroendocrine tumors. Diagnostics (Basel, Switzerland) 10:881. https://doi.org/10.3390/diagnostics10110881
Giovinazzo F, Schimmack S, Svejda B et al (2013) Chromogranin A and its fragments as regulators of small intestinal neuroendocrine neoplasm proliferation. PLoS One 8:e81111. https://doi.org/10.1371/journal.pone.0081111
Corsello A, Di Filippo L, Massironi S et al (2018) Vasostatin-1: A novel circulating biomarker for ileal and pancreatic neuroendocrine neoplasms. PLoS ONE 13:e0196858. https://doi.org/10.1371/journal.pone.0196858
Sherman SK, Maxwell JE, O’Dorisio MS et al (2014) Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol 21:2971–2980. https://doi.org/10.1245/s10434-014-3728-0
Andreasi V, Partelli S, Manzoni M et al (2019) Association between preoperative Vasostatin-1 and pathological features of aggressiveness in localized nonfunctioning pancreatic neuroendocrine tumors (NF-PanNET). Pancreatology 19:57–63. https://doi.org/10.1016/j.pan.2018.11.005
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527. https://doi.org/10.1136/bmj.h5527
Crippa L, Bianco M, Colombo B et al (2013) A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 121:392–402. https://doi.org/10.1182/blood-2012-05-430314
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Rindi G, Kloppel G, Alhman H et al (2006) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449:395–401. https://doi.org/10.1007/s00428-006-0250-1
Lloyd RV, Osamura RY, Kloppel G, Rosai J (2017) WHO Classification of Tumours of Endocrine Organs, 4th edn. IARC Press, Lyon
Oberg K, Modlin IM, De Herder W et al (2015) Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol 16:e435–e446. https://doi.org/10.1016/S1470-2045(15)00186-2
Tran CG, Sherman SK, Scott AT et al (2021) It is time to rethink biomarkers for surveillance of small bowel neuroendocrine tumors. Ann Surg Oncol 28:732–741. https://doi.org/10.1245/s10434-020-08784-0
Woltering EA, Voros BA, Beyer DT et al (2019) Plasma pancreastatin predicts the outcome of surgical cytoreduction in neuroendocrine tumors of the small bowel. Pancreas 48:356–362. https://doi.org/10.1097/MPA.0000000000001263
Pulvirenti A, Rao D, Mcintyre CA et al (2019) Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB (Oxford) 21:612–618. https://doi.org/10.1016/j.hpb.2018.09.016
Tseng C-M, Cheng T-Y, Chen T-B et al (2018) Low accuracy of chromogranin A for diagnosing early-stage pancreatic neuroendocrine tumors. Oncol Lett 15:8951–8958. https://doi.org/10.3892/ol.2018.8472
Qiu W, Christakis I, Silva A et al (2016) Utility of chromogranin A, pancreatic polypeptide, glucagon and gastrin in the diagnosis and follow-up of pancreatic neuroendocrine tumours in multiple endocrine neoplasia type 1 patients. Clin Endocrinol (Oxf) 85:400–407. https://doi.org/10.1111/cen.13119
Strosberg D, Schneider EB, Onesti J et al (2018) Prognostic impact of serum pancreastatin following chemoembolization for neuroendocrine tumors. Ann Surg Oncol 25:3613–3620. https://doi.org/10.1245/s10434-018-6741-x
Woltering EA, Beyer DT, Thiagarajan R et al (2016) Elevated plasma pancreastatin, but not chromogranin a, predicts survival in neuroendocrine tumors of the duodenum. J Am Coll Surg 222:534–542. https://doi.org/10.1016/j.jamcollsurg.2015.12.014
Pavel M, Jann H, Prasad V et al (2017) NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology 104:170–182. https://doi.org/10.1159/000446025
Laskaratos F-M, Liu M, Malczewska A et al (2020) Evaluation of circulating transcript analysis (NETest) in small intestinal neuroendocrine neoplasms after surgical resection. Endocrine 69:430–440. https://doi.org/10.1007/s12020-020-02289-2
Modlin IM, Kidd M, Frilling A et al (2021) Molecular genomic assessment using a blood-based mRNA signature (NETest) is cost effective and predicts neuroendocrine tumor recurrence with 94% accuracy. Ann Surg 274:481–490. https://doi.org/10.1097/SLA.0000000000005026
Modlin IM, Kidd M, Oberg K et al (2021) Early identification of residual disease after neuroendocrine tumor resection using a liquid biopsy multigenomic mRNA signature (NETest). Ann Surg Oncol 28:7506–7517. https://doi.org/10.1245/s10434-021-10021-1
Oberg K, Krenning E, Sundin A et al (2016) A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect 5:174–187. https://doi.org/10.1530/EC-16-0043
Acknowledgements
The authors are grateful to Gioja Bianca Costanza Fund for supporting the PhD Scholarship of Dr Valentina Andreasi and the Research Fellowship of Dr Francesca Muffatti. The present study was supported by ENETS Translational Medicine Fellowship Grant 2019. The authors thank the ERN EURACAN initiative.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design: VA, SP, AC, and MF. Data collection: VA, FM, and LDF. Analysis of the data: VA and SP. Interpretation of the data: VA, SP, MFM, SC, AC, and MF. Drafting the manuscript: VA and SP. Critical revision of the manuscript: all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The present study was approved by the San Raffaele Scientific Institute Ethics Committee (protocol NETBANK001).
Research involving human participants
All procedures performed in human participants were in accordance with the ethical standards of the institutional ethics committee and with the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from all the participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andreasi, V., Partelli, S., Manzoni, M.F. et al. Role of chromogranin A-derived fragments after resection of nonfunctioning pancreatic neuroendocrine tumors. J Endocrinol Invest 45, 1209–1217 (2022). https://doi.org/10.1007/s40618-022-01750-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01750-5