Abstract
Purpose
Early institution of GH therapy in children with Prader–Willi syndrome (PWS) yields beneficial effects on their phenotype and is associated with a persistent improvement of body composition, both in the transition age and in adulthood. Reports from GH stimulation testing in PWS adults, however, suggest that GH deficiency (GHD) is not a universal feature of the syndrome, and the current Consensus Guidelines suggest to perform a reassessment of persistent GHD so as to continue GH therapy after reaching adult height. Few data about GH responsiveness to stimulation testing throughout the transitional period in PWS are available to date. Thus, we investigated the prevalence of GHD in a large cohort of patients with PWS during the transition phase.
Patients and methods
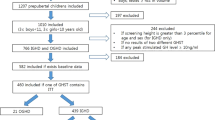
One hundred forty-one PWS patients, 72 females and 69 males, aged 15.4–24.9 years, were evaluated by dynamic testing with growth hormone‐releasing hormone (GHRH) plus arginine (GHRH + ARG). To define GHD, both BMI-dependent and BMI-independent diagnostic cut-off limits were considered.
Results
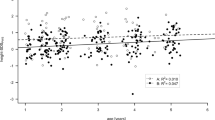
According to BMI‐dependent criteria, 10.7% of normal weight (NW), 18.5% of overweight and 22.1% of obese PWS maintained a status of GHD. Similar results were obtained by adopting a cut-off limit specific for the adult age (26.2%), as well as criteria for the transition phase in NW subjects (25%).
Conclusion
Our study shows that about 20% of patients with PWS fulfilled the criteria for GHD during the transitional age, suggesting the need of an integrated analysis of GH/IGF-I axis, in the context of the general clinical picture and other endocrine abnormalities, in all subjects after attainment of final stature.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ (2012) Prader-Willi syndrome. Genet Med 14:10–26
Butler MG, Hartin SN, Hossain WA, Manzardo AM, Kimonis V, Dykens E, Gold JA, Kim SJ, Weisensel N, Tamura R, Miller JL, Driscoll DJ (2019) Molecular genetic classification in Prader-Willi syndrome: a multisite cohort study. J Med Genet 56:149–153
Bochukova EG, Lawler K, Croizier S, Keogh JM, Patel N, Strohbehn G, Lo KK, Humphrey J, Hokken-Koelega A, Damen L, Donze S, Bouret SG, Plagnol V, Farooqi IS (2018) A transcriptomic signature of the hypothalamic response to fasting and BDNF deficiency in Prader-Willi Syndrome. Cell Rep 22:3401–3408
Angulo MA, Butler MG, Cataletto ME (2015) Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38:1249–1263
Grugni G, Marzullo P (2016) Diagnosis and treatment of GH deficiency in Prader-Willi syndrome. Best Pract Res Clin Endocrinol Metab 30:785–794
Marostica E, Grugni G, De Nicolao G, Marazzi N, Crinò A, Cappa M, Sartorio A (2013) The GHRH + arginine stimulated pituitary GH secretion in children and adults with Prader-Willi syndrome shows age- and BMI-dependent and genotype-related differences. Growth Horm IGF Res 23:261–266
Corrias A, Grugni G, Crinò A, Di Candia S, Chiabotto P, Cogliardi A, Chiumello G, De Medici C, Spera S, Gargantini L, Iughetti L, Luce A, Mariani B, Ragusa L, Salvatoni A, Andrulli S, Mussa A, Beccaria L, Study Group for Genetic Obesity of Italian Society of Pediatric Endocrinology and Diabetology (SIEDP/ISPED) (2012) Assessment of central adrenal insufficiency in children and adolescents with Prader-Willi syndrome. Clin Endocrinol (Oxf) 76:843–850
Cohen M, Harrington J, Narang I, Hamilton J (2015) Growth hormone secretion decreases with age in pediatric Prader-Willi syndrome. Clin Endocrinol (Oxf) 83:212–215
Sbardella E, Pozza C, Isidori AM, Grossman AB (2019) ENDOCRINOLOGY AND ADOLESCENCE: Dealing with transition in young patients with pituitary disorders. Eur J Endocrinol 181:R155–R171
Kazemi E, Hodapp RM (2006) Transition from adolescence to young adulthood: the special case of Prader-Willi syndrome. In: Butler MG, Lee PDK, Whitman Y (eds) Management of Prader-Willi syndrome, 3rd edn. Springer, Berlin, pp 356–369
Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M, Speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS (2008) Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 93:4183–4197
Grugni G, Sartorio A, Crinò A (2016) Growth hormone therapy for Prader-Willi syndrome: challenges and solutions. Ther Clin Risk Manag 12:873–881
Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M (2005) Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 152:165–170
Donze SH, Damen L, Alfen-van der Velden JAEM, Bocca G, Finken MJJ, Hoorweg-Nijman GJG, Jira PE, van Leeuwen M, Hokken-Koelega ACS (2019) Prevalence of growth hormone (GH) deficiency in previously GH-treated young adults with Prader-Willi syndrome. Clin Endocrinol (Oxf) 91:118–123
Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G, Cicognani A (2006) Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 29:581–593
Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Körner A, Obermayer-Pietsch B, Hübener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM (2014) Reference intervals for insulin-like growth factor-1 (IGFI) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab 99:1712–1721
Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, Ghigo E, Camanni F, Aimaretti G (2005) The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol 153:257–264
Aimaretti G, Baffoni C, Bellone S, Di Vito L, Corneli G, Arvat E, Benso L, Camanni F, Ghigo E (2000) Retesting young adults with childhood-onset growth hormone (GH) deficiency with GH-releasing-hormone-plus-arginine test. J Clin Endocrinol Metab 85:3693–3699
Corneli G, Di Somma C, Prodam F, Bellone J, Bellone S, Gasco V, Baldelli R, Rovere S, Schneider HJ, Gargantini L, Gastaldi R, Ghizzoni L, Valle D, Salerno M, Colao A, Bona G, Ghigo E, Maghnie M, Aimaretti G (2007) Cut-off limits of the GH response to GHRH plus arginine test and IGF-I levels for the diagnosis of GH deficiency in late adolescents and young adults. Eur J Endocrinol 157:701–708
Aimaretti G, Corneli G, Razzore P, Bellone S, Baffoni C, Arvat E, Camanni F, Ghigo E (1998) Comparison between insulin-induced hypoglycemia and growth hormone (GH)-releasing hormone+arginine as provocative tests for the diagnosis of GH deficiency in adults. J Clin Endocrinol Metab 83:1615–1618
Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH, Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society (2016) Guidelines for Growth Hormone and Insulin-Like Growth Factor-I treatment in children and adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm Res Paediatr 86:361–397
Cook DM, Rose SR (2012) A review of guidelines for use of growth hormone in pediatric and transition patients. Pituitary 15:301–310
Patti G, Noli S, Capalbo D, Allegri AME, Napoli F, Cappa M, Ubertini GM, Gallizia A, Notarnicola S, Ibba A, Crocco M, Parodi S, Salerno M, Loche S, Garré ML, Tornari E, Maghnie M, Di Iorgi N (2019) Accuracy and limitations of the Growth Hormone (GH) Releasing Hormone-arginine retesting in young adults with childhood-onset GH deficiency. Front Endocrinol (Lausanne) 10:525
Höybye C, Tauber M, Angulo MA, Eiholzer U, Driscoll DJ, Cassidy SB, Holland AJ (2019) Letter regarding “Prevalence of growth hormone deficiency in previously GH-treated young adults with Prader-Willi syndrome” by Donze et al. Clin Endocrinol (Oxf) 91:578–579
Diene G, Mimoun E, Feigerlova E, Caula S, Molinas C, Grandjean H, Tauber M, French Reference Centre for PWS (2010) Endocrine disorders in children with Prader-Willi syndrome: data from 142 children of the French database. Horm Res Paediatr 74:121–128
Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS, 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants (2013) Growth Hormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 98:E1072-1087
Di Giorgio G, Grugni G, Fintini D, Bocchini S, Spera S, Cuttini M, Cappa M, Crinò A (2014) Growth hormone response to standard provocative stimuli and combined tests in very young children with Prader-Willi syndrome. Horm Res Paediatr 81:189–195
Maghnie M, Aimaretti G, Bellone S, Bona G, Bellone J, Baldelli R, de Sanctis C, Gargantini L, Gastaldi R, Ghizzoni L, Secco A, Tinelli C, Ghigo E (2005) Diagnosis of GH deficiency in the transition period: accuracy of insulin tolerance test and insulin-like growth factor-I measurement. Eur J Endocrinol 152:589–596
Iughetti L, Bosio L, Corrias A, Gargantini L, Ragusa L, Livieri C, Predieri B, Bruzzi P, Caselli G, Grugni G (2008) Pituitary height and neuroradiological alterations in patients with Prader-Labhart-Willi syndrome. Eur J Pediatr 167:701–702
van Nieuwpoort IC, Sinnema M, Castelijns JA, Twisk JW, Curfs LM, Drent ML (2011) The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm Res Paediatr 75:403–411
Oto Y, Tanaka Y, Abe Y, Obata K, Tsuchiya T, Yoshino A, Murakami N, Nagai T (2014) Exacerbation of BMI after cessation of growth hormone therapy in patients with Prader-Willi syndrome. Am J Med Genet A 164A:671–675
Coupaye M, Tauber M, Cuisset L, Laurier V, Bieth E, Lacorte JM, Oppert JM, Clément K, Poitou C (2016) Effect of genotype and previous GH treatment on adiposity in adults with Prader-Willi syndrome. J Clin Endocrinol Metab 101:4895–4903
Paepegaey AC, Coupaye M, Jaziri A, Ménesguen F, Dubern B, Polak M, Oppert JM, Tauber M, Pinto G, Poitou C (2018) Impact of transitional care on endocrine and anthropometric parameters in Prader-Willi syndrome. Endocr Connect 7:663–672
Sode-Carlsen R, Farholt S, Rabben KF, Bollerslev J, Schreiner T, Jurik AG, Christiansen JS, Höybye C (2012) Growth hormone treatment in adults with Prader-Willi syndrome: the Scandinavian study. Endocrine 41:191–199
Kuppens RJ, Bakker NE, Siemensma E, Tummers-de Lind van Wijngaarden RF, Donze SH, Festen DA, Alfen-van der Velden JA, Stijnen T, Hokken-Koelega AC (2016) Beneficial effects of GH in young adults with Prader-Willi Syndrome: a 2-Year crossover trial. J Clin Endocrinol Metab 101:4110–4116
Damen L, Grootjen LN, Donze SH, Juriaans AF, de Graaff LCG, van der Velden JAEM, Hokken-Koelega ACS (2020) Three years of growth hormone treatment in young adults with Prader-Willi Syndrome previously treated with growth hormone in childhood: Effects on glucose homeostasis and metabolic syndrome. Clin Endocrinol (Oxf). https://doi.org/10.1111/cen.14274
Kuppens RJ, Mahabier EF, Bakker NE, Siemensma EP, Donze SH, Hokken-Koelega AC (2016) Effect of cessation of GH treatment on cognition during transition phase in Prader-Willi syndrome: results of a 2-year crossover GH trial. Orphanet J Rare Dis 11:153
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine Society (2011) Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1587–1609
Ho KKY on behalf of the 2007 GH Deficiency Consensus Workshop Participants (2007) Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157:695–700
Secco A, di Iorgi N, Napoli F, Calandra E, Calcagno A, Ghezzi M, Frassinetti C, Fratangeli N, Parodi S, Benassai M, Leitner Y, Gastaldi R, Lorini R, Maghnie M, Radetti G (2009) Reassessment of the growth hormone status in young adults with childhood-onset growth hormone deficiency: reappraisal of insulin tolerance testing. J Clin Endocrinol Metab 94:4195–4204
Casamitjana L, Giménez-Palop O, Corripio R, Pareja R, Berlanga E, Rigla M, Oliva JC, Caixàs A (2020) Glucagon stimulation test to assess growth hormone status in Prader-Willi syndrome. J Endocrinol Invest. https://doi.org/10.1007/s40618-020-01367-6
Crinò A, Fintini D, Bocchini S, Carducci C, Grugni G (2016) Prader-Willi syndrome: clinical problems in transition from pediatric to adult care. Res Rep Endocr Dis 6:49–57
Acknowledgements
We are grateful to the patients with PWS and their families for their willingness to participate in this research. Our special thanks to the Italian Prader–Willi Syndrome Association for its collaboration.
Funding
This work was partially supported by Progetti di Ricerca Corrente, Istituto Auxologico Italiano, IRCCS, Milan, Italy (ref. no 01C629; acronym: PWStransGH).
Author information
Authors and Affiliations
Consortia
Contributions
GG designed the study. GG, MD, LI, SO, LR, AS, SS and AC recruited the patients. GG and PM analyzed the data and wrote the original draft of the manuscript. MD, LI, MRL, SO, LR, AS, AS, SS and AC discussed the results and contributed to additional versions of the manuscript. AS and PM performed writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors have nothing to disclose.
Ethical approval
The protocol was run in accordance with the Declaration of Helsinki as revised in 2008. The study was approved by the Ethical Committee of the Istituto Auxologico Italiano (ref. no 01C629; acronym: PWStransGH).
Consent to participate
Written informed consent was obtained for all procedures from the parents or legal representatives, and from the patients when applicable.
Consent for publication
All authors have read and agreed to publish the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grugni, G., Marzullo, P., Delvecchio, M. et al. Stimulated GH levels during the transition phase in Prader–Willi syndrome. J Endocrinol Invest 44, 1465–1474 (2021). https://doi.org/10.1007/s40618-020-01450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01450-y