Abstract
Purpose
Cold exposure activates the hypothalamus–pituitary–thyroid (HPT) axis, response blunted by previous acute stress or corticosterone administration. Chronic stressors can decrease serum T3 concentration, and thyrotropin-releasing hormone (Trh) expression in the paraventricular nucleus (PVN), but impact on the response to cold is unknown; this was studied in rats submitted to daily repeated restraint (rRes) that causes habituation of hypothalamus–pituitary–adrenal (HPA) axis response, or to chronic variable stress (CVS) that causes sensitization and hyperreactivity.
Methods
Wistar male adult rats were submitted to rRes 30 min/day, or to CVS twice a day, for 15 days. On day 16, rats were exposed 1 h to either 5 or 21 °C. Parameters of HPT and HPA axes activity and of brown adipose tissue (BAT) cold response were measured; gene expression in PVN and BAT, by RT-PCR; serum hormone concentration by radioimmunoassay or ELISA.
Results
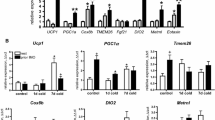
Compared to naïve animals, Crh and corticosterone concentrations were attenuated at the end of rRes, but increased at the end of CVS treatments. Cold exposure increased mRNA levels of Crh, Trh, and serum concentration of thyrotropin in naïve, but not in rRes or CVS rats; corticosterone increased in all groups. Cold induced expression of thermogenic genes in BAT (Dio2 and Ucp1) in naïve but not in stressed rats; Adrb3 expression was differentially regulated.
Conclusion
Both types of chronic stress blunted HPT and BAT responses to cold. Long-term stress effects on noradrenergic and/or hormonal signaling are likely responsible for HPT dysfunction and not the type of chronic stressor.




Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropin
- Adrb3 :
-
β3 Adrenergic receptor gene
- BAT:
-
Brown adipose tissue
- BWg:
-
Body weight gain
- cAMP:
-
Cyclic adenosine monophosphate
- Cort:
-
Corticosterone
- Cpf:
-
Control pair-fed
- Crh :
-
Corticotropin-releasing hormone gene
- CRH:
-
Corticotropin-releasing hormone peptide
- CVS:
-
Chronic variable stress
- Dio2 :
-
Deiodinase 2 gene
- DIO2:
-
Deiodinase 2
- EPM:
-
Elevated plus maze
- Gr:
-
Glucocorticoid receptor gene (Nr3c1: nuclear receptor subfamily 3 group C member gene)
- HPA:
-
Hypothalamus–pituitary–adrenal axis
- HPT:
-
Hypothalamus–pituitary–thyroid axis
- N:
-
Naïve
- OFT:
-
Open field test
- pCREB:
-
Phosphorylated cAMP response element-binding protein
- PKA:
-
Protein kinase A
- PKAc:
-
Catalytic subunit of PKA
- PVN:
-
Paraventricular nucleus of the hypothalamus
- rRes:
-
Repeated restraint stress
- RT:
-
Room temperature
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SAS:
-
Sympathoadrenal system
- T3:
-
3, 3′, 5-Triiodo-l-thyronine
- T4:
-
Thyroxine
- Trh :
-
Thyrotropin-releasing hormone gene
- TRH:
-
Thyrotropin-releasing hormone peptide
- TSH:
-
Thyrotropin
- Ucp1 :
-
Uncoupling protein 1 gene
- UCP-1:
-
Uncoupling protein 1
- WAT:
-
White adipose tissue
References
Morrison SF (2018) Efferent neural pathways for the control of brown adipose tissue thermogenesis and shivering. Handb Clin Neurol 156:281–303. https://doi.org/10.1016/B978-0-444-63912-7.00017-5
Nedergaard J, Cannon B (2018) Brown adipose tissue as a heat-producing thermoeffector. Handb Clin Neurol 156:137–152. https://doi.org/10.1016/B978-0-444-63912-7.00009
Rondeel JM, de Greef WJ, Hop WC, Rowland DL, Visser TJ (1991) Effect of cold exposure on the hypothalamic release of thyrotropin-releasing hormone and catecholamines. Neuroendocrinology 54:477–481. https://doi.org/10.1159/000125940
Hefco E, Krulich L, Illner P, Larsen PR (1975) Effect of acute exposure to cold on the activity of the hypothalamic-pituitary-thyroid system. Endocrinology 97:1185–1195. https://doi.org/10.1210/endo-97-5-1185
Uribe RM, Redondo JL, Charli JL, Joseph-Bravo P (1993) Suckling and cold stress rapidly and transiently increase TRH mRNA in the paraventricular nucleus. Neuroendocrinology 58:140–145. https://doi.org/10.1159/000126523
Perello M, Stuart RC, Vaslet CA, Nillni EA (2007) Cold exposure increases the biosynthesis and proteolytic processing of prothyrotropin-releasing hormone in the hypothalamic paraventricular nucleus via beta-adrenoreceptors. Endocrinology 148:4952–4964. https://doi.org/10.1210/en.2007-0522
Bianco AC, Dumitrescu A, Gereben B et al (2019) Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev 40:1000–1047. https://doi.org/10.1210/er.2018-00275
Martinez-deMena R, Anedda A, Cadenas S, Obregon MJ (2015) TSH effects on thermogenesis in rat brown adipocytes. Mol Cell Endocrinol 404:151–158. https://doi.org/10.1016/j.mce.2015.01.028
Herman JP, McKlveen JM et al (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp Physiol 6:603–621. https://doi.org/10.1002/cphy.c150015
Fukuhara K, Kvetnansky R, Cizza G, Pacak K, Ohara H, Goldstein DS, Kopin IJ (1996) Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold tress. J Neuroendocrinol 8:533–541. https://doi.org/10.1046/j.1365-2826.1996.04877
Joseph-Bravo P, Jaimes-Hoy L, Charli JL (2015) Regulation of TRH neurons and energy homeostasis-related signals under stress. J Endocrinol 224:139–159. https://doi.org/10.1530/JOE-14-0593
Joseph-Bravo P, Jaimes-Hoy L, Charli JL (2016) Advances in TRH signaling. Rev Endocr Metab Disord 17:545–558. https://doi.org/10.1007/s11154-016-9375-y
Osterlund C, Spencer R (2011) Corticosterone pretreatment suppresser stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action and de novo protein synthesis. J Endocrinol 208:311–322. https://doi.org/10.1530/JOE-10-0413
Sotelo-Rivera I, Jaimes-Hoy L, Cote-Vélez A, Espinoza-Ayala C, Charli JL, Joseph-Bravo P (2014) An acute injection of corticosterone increases thyrotropin-releasing hormone expression in the paraventricular nucleus of the hypothalamus but interferes with the rapid hypothalamus pituitary thyroid axis response to cold in male rats. J Endocrinol 26:861–869. https://doi.org/10.1111/jne.12224
Sotelo-Rivera I, Cote-Vélez A, Uribe RM, Charli JL, Joseph-Bravo P (2017) Glucocorticoids curtail stimuli-induced CREB phosphorylation in TRH neurons trough interaction of the glucocorticoid receptor with the catalytic subunit of protein kinase A. Endocrine 55:861–871. https://doi.org/10.1007/s12020-016-1223-z
Kvetnansky R, Sabban EL, Palkovits M (2009) Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89:535–606. https://doi.org/10.1152/physrev.00042.2006
Herman JP, Tasker JG (2016) Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol 7:137. https://doi.org/10.3389/fendo.2016.00137
Radley JJ, Sawchenko PE (2015) Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol 523:2769–2787. https://doi.org/10.1002/cne.23815
Gutiérrez-Mariscal M, Sánchez E, García-Vázquez A, Rebolledo-Solleiro D, Charli JL, Joseph-Bravo P (2012) Acute response of hypophysiotropic thyrotropin releasing hormone neurons and thyrotropin release to behavioral paradigms producing varying intensities of stress and physical activity. Regul Pep 179:61–70. https://doi.org/10.1016/j.regpep.2012.08.010
Uribe RM, Jaimes-hoy L, Ramírez-Martínez C, García-Vazquez A, Romero F, Cisneros M, Cote-Vélez A, Charli JL, Joseph-Bravo P (2014) Voluntary exercise adapts the hypothalamus-pituitary-thyroid axis in male rats. Endocrinology 155:2020–2030. https://doi.org/10.1210/en.2013-1724
Armario A, García-Márquez C, Jolin T (1987) The effects of chronic intermittent stress on basal and acute stress levels of TSH and GH, and their response to hypothalamic regulatory factors in the rat. Psychoneuroendocrinology 12:399–406. https://doi.org/10.1016/0306-4530(87)90069-2
Parra-Montes de Oca MA, Gutiérrez-Mariscal M, Salmerón-Jiménez MF, Jaimes-Hoy L, Charli JL, Joseph-Bravo P (2019) Voluntary exercise-induced activation of the thyroid axis and reduction of white fat depots is attenuated by chronic stress in a sex dimorphic pattern in adult rats. Front Endocrinol 10:418. https://doi.org/10.3389/fendo.2019.00418
Fekete C, Lechan RM (2014) Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev 35:159–194. https://doi.org/10.1210/er.2013-1087
Brudzynski SM (2013) Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23:310–317. https://doi.org/10.1016/j.conb.2013.01.014
Harris RBS, Jun Z, Youngblood BD, Rybkin I, Smagin GN, Ryan DH (1998) Effect of repeated stress on body weight and body composition of rats fed low and high-fat diets. Am J Physiol 275:R1928–1938. https://doi.org/10.1152/ajpregu.1998.275.6.R1928
Flack JN, Jankord R, Solomon MB, Krause EG, Herman JP (2011) Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104:228–234. https://doi.org/10.1016/j.physbeh.2011.03.002
Ma XM, Lightman SL, Aguilera G (1999) Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology 140:3623–3632. https://doi.org/10.1210/endo.140.8.6943
Lowrance SA, Ionadi A, Douglas MEX, Johnson JD (2016) Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 68:163–170. https://doi.org/10.1016/j.psyneuen.2016.02.027
Di S, Malcher-Lopes R, Halmos KC, Tasker JG (2003) Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857. https://doi.org/10.1523/JNEUROSCI.23-12-04850.2003
Farkas E, Varga E, Kovács B, Szilvásy-Szabó A, Cote-Vélez A, Péterfi Z, Matziari M, Tóth M, Zelena D, Mezriczky Z, Kádár A, Kővári D, Watanabe M, Kano M, Mackie K, Rózsa B, Ruska Y, Tóth B, Máté Z, Erdélyi F, Szabó G, Gereben B, Lechan RM, Charli JL, Joseph-Bravo P, Fekete C (2020) A glial-neuronal circuit in the median eminence regulates thyrotropin-releasing hormone-release via the endocannabinoid system. iScience 23:100921. https://doi.org/10.1016/j.isci.2020.100921
John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC (2003) Kinase-dependent regulation of the secretion of thyrotrophin and luteinizing hormone by glucocorticoids and annexin 1 peptides. J Neuroendocrinol 15:946–957. https://doi.org/10.1046/j.1365-2826.2003.01081
Cavalieri RR, Castle JN, McMahon FA (1984) Effects of dexamethasone on kinetics and distribution of triiodothyronine in the rat. Endocrinology 114:215–221. https://doi.org/10.1210/endo-114-1-215
Hotta H, Onda A, Suzuki H, Milliken P, Sridhar A (2017) Modulation of calcitonin, parathyroid hormone, and thyroid hormone secretion by electrical stimulation of sympathetic and parasympathetic nerves in anesthetized rats. Front Neurosci 11:375. https://doi.org/10.3389/fnins.2017.00375
Oka T (2018) Stress-induced hyperthermia and hypothermia. Handb Clin Neurol 157:599–621. https://doi.org/10.1016/B978-0-444-64074-1.00035-5
Martinez-de Mena R, Calvo RM, Garcia L, Obregon MJ (2016) Effect of glucocorticoids on the activity, expression and proximal promoter of type II deiodinase in rat brown adipocytes. Mol Cel Endocrinol 428:58–67. https://doi.org/10.1016/j.mce.2016.03.021
Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand E, Silva JE (2000) Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cel Endocrinol 165:7–15. https://doi.org/10.1016/s0303-7207(00)00276-8
Ramage LE, Akyol M, Fletcher AM et al (2016) Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 24:130–141. https://doi.org/10.1016/j.cmet.2016.06.0141
Valle A, García-Palmer FJ, Oliver J, Roca P (2007) Sex differences in brown adipose tissue thermogenic features during caloric restriction. Cell Physiol Biochem 19:195–204. https://doi.org/10.1159/000099207
Laukova M, TillingerA Novakova M, Krizanova O, Kvetnansky R, Myslivcek J (2014) Repeated immobilization stress increases expression of beta3 adrenoreceptor in the left ventricle and atrium of the rat heart. Stress Health 30:301–309. https://doi.org/10.1002/smi.2515
Bengtsson T, Cannon B, Nedergaard J (2000) Differential adrenergic regulation of the gene expression of the beta-adrenoceptor subtypes beta1, beta2 and beta3 in brown adipocytes. Biochem J 347:643–651. https://doi.org/10.1042/0264-6021:3470643
Ong FJ, Ahmed BA, Oreskovich SM et al (2018) Recent advances in the detection of brown adipose tissue in adult humans: a review. Clin Sci (Lond) 132:1039–1054. https://doi.org/10.1042/CS20170276
Santhanam P, Ahima RS, Mammen JS, Giovanella L, Treglia G (2018) Brown adipose tissue (BAT) detection by 18F-FDG PET and thyroid hormone level(s)-a systematic review. Endocrine 62:496–500. https://doi.org/10.1007/s12020-018-1698-x
Heinen CA, Zhang Z, Klieverik LP et al (2018) Effects of intravenous thyrotropin-releasing hormone on 18F-fluorodeoxyglucose uptake in human brown adipose tissue: a randomized controlled trial. Eur J Endocrinol 179:31–38. https://doi.org/10.1530/EJE-17-0966
Leppäluoto J, Pääkkönen T, Korhonen I, Hassi J (2005) Pituitary and autonomic responses to cold exposures in man. Acta Physiol Scand 184:255–264. https://doi.org/10.1111/j.1365-201X.2005.01464.x
Fischer S, Ehlert U (2018) Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. Syst Rev Depress Anxiety 35:98–110. https://doi.org/10.1002/da.22692
Johansson G, Laakso ML, Karonen SL, Peder M (1987) Examination stress affects plasma levels of TSH and thyroid hormones differently in females and males. Psychosom Med 49:390–396. https://doi.org/10.1097/00006842-198707000-00008
Vogel WV, Valdés Olmos RA, Tijs TJ, Gillies MF, van Elswijk G, Vogt J (2012) Intervention to lower anxiety of 18F-FDG PET/CT patients by use of audiovisual imagery during the uptake phase before imaging. J Nucl Med Technol 40:92–98. https://doi.org/10.2967/jnmt.111.097964
Acknowledgements
The authors acknowledge the technical assistance of M. Gutiérrez-Mariscal, M. Cisneros, F. Romero, R. Rodríguez Bahena, S. Ainsworth and E. López Bustos.
Funding
The present study was supported by UNAM-DGAPA (Grant no. IN213419), CONACYT (Schollarships for graduate studies), CONACYT (Grant no. 284883).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Maintenance and work with animals followed the Guide for the care and use of laboratory animals (8th ed.), as well as the Mexican norm NOM-062-ZOO-1999. These experiments were approved by the Bioethics Committee of the Institute, approval No. 273 and 318.
Informed consent
For these animal studies consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castillo-Campos, A., Gutiérrez-Mata, A., Charli, JL. et al. Chronic stress inhibits hypothalamus–pituitary–thyroid axis and brown adipose tissue responses to acute cold exposure in male rats. J Endocrinol Invest 44, 713–723 (2021). https://doi.org/10.1007/s40618-020-01328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01328-z