Abstract
Purpose
Adversity in early life can induce metabolic defects in exposure to stress in adulthood. Therefore, the exploration of involving mechanisms can be helpful in the treatment of metabolic disorders. So, the present study was conducted in terms of exploring the effects of interaction between early postnatal stress and young adulthood psychological stress on insulin secretion and pancreatic GLUT-2 levels in male rats.
Methods
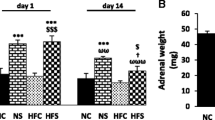
Footshock as a model of early life stress (at 2 weeks of age) and psychological stress induced by communication box as a model of young adulthood stress (at 8–10 weeks of age) were induced in male Wistar rats for five consecutive days (2 times/day). Blood samples were drawn to measure glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell dysfunction (HOMA-B), before and after stress protocol in young adult rats. Corticosterone was measured on days 1 and 5 of stress induction. The day after the stress period, factors including glucose tolerance, TNF-alpha, isolated islets’ insulin output and levels of pancreatic GLUT-2 protein via western blotting were determined.
Results
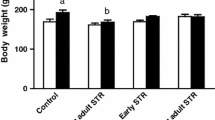
The combination of early footshock exposure and psychological stress during adulthood did not affect plasma corticosterone, but increased plasma insulin, HOMA-IR, HOMA-B and TNF-alpha levels. Plasma TNF was not only increased by the combination of both stressors, but also after only E STR exposure. HOMA-IR was increased in both Psy STR and E + Psy-STR groups. Plasma glucose just increased in Psy STR group. The combination of these two life stressors further increased the in vitro insulin secretion from isolated islets in response to 16.7-mM glucose. The level of Glut2 was increased in Psy STR and decreased in both E STR and E + Psy STR groups. Finally, glucose tolerance was impaired and glucose-stimulated insulin secretion was increased in E + Psy STR group.
Conclusions
In conclusion, inducing stress in early life makes the organism more susceptible to metabolic defects in exposure to psychological stress later in life.





Similar content being viewed by others
References
Friedman EM, Karlamangla AS, Gruenewald T, Koretz B, Seeman TE (2015) Early life adversity and adult biological risk profiles. Psychosom Med 77(2):176
Ilchmann-Diounou H, Olier M, Lencina C, Riba A, Barretto S, Nankap M, Sommer C, Guillou H, Ellero-Simatos S, Guzylack-Piriou L (2019) Early life stress induces type 2 diabetes-like features in ageing mice. Brain Behav Immun 80:452–463
Ruiz R, Roque A, Pineda E, Licona-Limón P, Valdéz-Alarcón JJ, Lajud N (2018) Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 96:203–211
Vargas J, Junco M, Gomez C, Lajud N (2016) Early life stress increases metabolic risk, HPA axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats. PLoS ONE 11(9):e0162665
Santarelli S, Zimmermann C, Kalideris G, Lesuis SL, Arloth J, Uribe A, Dournes C, Balsevich G, Hartmann J, Masana M (2017) An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 78:213–221
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98(4):2133–2223
Nolan CJ, Prentki M (2019) Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: tTime for a conceptual framework shift. Diabetes Vasc Dis Res 16(2):118–127
Whirledge S, DeFranco DB (2017) Glucocorticoid signaling in health and disease: insights from tissue-specific GR knockout mice. Endocrinology 159(1):46–64
Pedersen JM, Mortensen EL, Christensen DS, Rozing M, Brunsgaard H, Meincke RH, Petersen GL, Lund R (2018) Prenatal and early postnatal stress and later life inflammation. Psychoneuroendocrinology 88:158–166
Pedersen JM, Lund R, Andersen I, Clark AJ, Prescott E, Rod NH (2016) Psychosocial risk factors for the metabolic syndrome: a prospective cohort study. Int J Cardiol 215:41–46
Levine M, Cole S, Weir D, Crimmins E (2015) Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med 130:16–22
Kim W-H, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, Song JH, Gao B, Jung MH (2005) Exposure to chronic high glucose induces β-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic β-cells. Diabetes 54(9):2602–2611
Navarro-Tableros V, Fiordelisio T, Hernández-Cruz A, Hiriart M (2007) Physiological development of insulin secretion, calcium channels, and GLUT2 expression of pancreatic rat β-cells. Am J Physiol-Endocrinol Metab 292(4):E1018–E1029
Farr OM, Ko B-J, Joung KE, Zaichenko L, Usher N, Tsoukas M, Thakkar B, Davis CR, Crowell JA, Mantzoros CS (2015) Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovas Dis 25(5):479–488
Nasca C, Watson-Lin K, Bigio B, Robakis TK, Myoraku A, Wroolie TE, McEwen BS, Rasgon N (2019) Childhood trauma and insulin resistance in patients suffering from depressive disorders. Exp Neurol 315:15–20
Zardooz H, Zahediasl S, Rostamkhani F, Farrokhi B, Nasiraei S, Kazeminezhad B, Gholampour R (2012) Effects of acute and chronic psychological stress on isolated islets’ insulin release. EXCLI J 11:163
Matsumoto M, Higuchi K, Togashi H, Koseki H, Yamaguchi T, Kanno M, Yoshioka M (2005) Early postnatal stress alters the 5-HTergic modulation to emotional stress at postadolescent periods of rats. Hippocampus 15(6):775–781
Andersen ML, Bignotto M, Machado RB, Tufik S (2004) Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res 37(6):791–797
Zardooz H, Asl SZ, Naseri MG (2006) Effect of chronic psychological stress on insulin release from rat isolated pancreatic islets. Life Sci 79(1):57–62
Oosterlinck W, Vanderper A, Flameng W, Herijgers P (2011) Glucose tolerance and left ventricular pressure-volume relationships in frequently used mouse strains. BioMed Res Int 2011:1–7
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Krzyzanowska K, Zemany L, Krugluger W, Schernthaner G, Mittermayer F, Schnack C, Rahman R, Brix J, Kahn B, Schernthaner G (2008) Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia 51(7):1115–1122
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16(1):35–39
Vélez-Granell CS, Arias AE, Torres-Ruíz JA, Bendayan M (1994) Molecular chaperones in pancreatic tissue: the presence of cpn10, cpn60 and hsp70 in distinct compartments along the secretory pathway of the acinar cells. J Cell Sci 107(3):539–549
Ladd CO, Huot RL, Thrivikraman K, Nemeroff CB, Plotsky PM (2004) Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiat 55(4):367–375
Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM (2007) Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25(10):3091–3098
Ladd CO, Thrivikraman K, Huot RL, Plotsky PM (2005) Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology 30(6):520–533
Eiland L, McEwen BS (2012) Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22(1):82–91
Fóscolo DRC, Fóscolo RB, Marubayashi U, Reis AM, Coimbra CC (2008) Neonatal maternal separation affects endocrine and metabolic stress responses to ether exposure but not to restraint exposure in adult rats. Metab Brain Dis 23(4):375
Wu XY, Hu YT, Guo L, Lu J, Zhu QB, Yu E, Wu JL, Shi LG, Huang ML, Bao AM (2015) Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav 145(1):118–121
De Boer S, Slangen J, Van der Gugten J (1988) Adaptation of plasma catecholamine and corticosterone responses to short-term repeated noise stress in rats. Physiol Behav 44(2):273–280
McPherson RJ, Mascher-Denen M, Juul SE (2009) Postnatal stress produces hyperglycemia in adult rats exposed to hypoxia-ischemia. Pediatr Res 66(3):278
Teague CR, Dhabhar FS, Barton RH, Beckwith-Hall B, Powell J, Cobain M, Singer B, McEwen BS, Lindon JC, Nicholson JK (2007) Metabonomic studies on the physiological effects of acute and chronic psychological stress in sprague− dawley rats. J Proteome Res 6(6):2080–2093
Eguchi R, Scarmagnani FR, Cunha CA, Souza GI, Pisani LP, Ribeiro EB, do Nascimento CMO, Spadari-Bratfisch RC, Oyama LM (2011) Fish oil consumption prevents glucose intolerance and hypercorticosteronemy in footshock-stressed rats. Lipids Health Dis 10(1):7
Wilcox G (2005) Insulin and insulin resistance. Clin Biochemist Rev 26(2):19
Wang Y, Nishi M, Doi A, Shono T, Furukawa Y, Shimada T, Furuta H, Sasaki H, Nanjo K (2010) Ghrelin inhibits insulin secretion through the AMPK–UCP2 pathway in β cells. FEBS Lett 584(8):1503–1508
Liu Y-Z, Wang Y-X, Jiang C-L (2017) Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci 11:316
Pruett SB (2003) Stress and the immune system. Pathophysiology 9(3):133–153
Li J, Bai L, Wei F, Zhao J, Wang D, Xiao Y, Yan W, Wei J (2019) Therapeutic mechanisms of herbal medicines against insulin resistance: a review. Front pharmacol 10:661
Pittas AG, Joseph NA, Greenberg AS (2004) Adipocytokines and insulin resistance. J Clin Endocrinol Metab 89(2):447–452
Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB (2012) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci 109(16):5995–5999
Hohmann CF, Odebode G, Naidu L, Koban M (2017) Early life stress alters adult inflammatory responses in a mouse model for depression. Ann Psychiatry Mental Health 5(2):1–12
Fagundes CP, Way B (2014) Early-life stress and adult inflammation. Curr Dir Psychol Sci 23(4):277–283
Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID (2008) Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology 149(6):2727–2736
Alvarez P, Green PG, Levine JD (2013) Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiat 74(9):688–695
Courty E, Besseiche A, Do TTH, Liboz A, Aguid FM, Quilichini E, Buscato M, Gourdy P, Gautier J-F, Riveline J-P (2019) Adaptive β-cell neogenesis in the adult mouse in response to glucocorticoid-induced insulin resistance. Diabetes 68(1):95–108
Guillam M-T, Hümmler E, Schaerer E, Wu J-Y, Birnbaum MJ, Beermann F, Schmidt A, Dériaz N, Thorens B (1997) Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 17(3):327
Tal M, Wu YJ, Leiser M, Surana M, Lodish H, Fleischer N, Weir G, Efrat S (1992) [Val12] HRAS downregulates GLUT2 in beta cells of transgenic mice without affecting glucose homeostasis. Proc Natl Acad Sci 89(13):5744–5748
De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F (1995) Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96(5):2489–2495
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73
Dhar A, Dhar I, Jiang B, Desai KM, Wu L (2011) Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 60(3):899–908
Acknowledgements
We thank Dr. Hossein Khaleghzadeh-Ahangar, the Assistant Professor at Babol University of Medical Sciences for his aid in English editing. This work was supported by Diabetes Research Center (Grant 3069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standards
All procedures were approved by the Animal Care and Use Committee of the Diabetes Research Center of Research Deputy of Mazandaran University of Medical Sciences, Sari, Iran.
Informed consent
For this type of study formal consent in not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zardooz, H., Sadeghimahalli, F. & Khodagholi, F. Early postnatal stress impairs insulin secretion in response to psychological stress in adult rats. J Endocrinol Invest 44, 277–286 (2021). https://doi.org/10.1007/s40618-020-01291-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01291-9