Abstract
Purpose
Under-carboxylated osteocalcin (UcOC), a bone-released hormone is suggested to regulate energy metabolism. Pregnancy and lactation physiological conditions that require high levels of energy. The current study attempts to examine whether UcOC is involved in regulating energy metabolism during these conditions using adult Wistar rats.
Methods and results
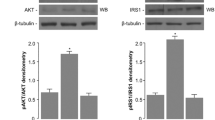
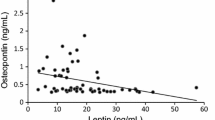
Insulin tolerance tests indicated insulin resistance during late pregnancy (day 19 of pregnancy; P19) and insulin sensitivity during early lactation (day 6 of lactation; L6). Gene expression analyses suggested that muscle glucose metabolism was downregulated during P19 and enhanced during L6. Concomitantly, circulatory UcOC levels were lower during pregnancy but higher during early lactation; the rise in UcOC levels was tightly linked to the lactation process. Altering endogenous UcOC levels pharmacologically with warfarin and alendronate in P19 and L6 rats changed whole-body insulin response and muscle glucose transporter (Glut4) expression. Glut4 expression can be increased by either UcOC or estrogen receptors (ERs), both of which act independent of each other. A high fat diet decreased UcOC levels and insulin sensitivity in lactating rats, suggesting that diet can compromise UcOC-established energy homeostasis. Gene expression of lipid metabolism markers and triglyceride levels suggested that UcOC suppression during early pregnancy is an essential step in maternal lipid storage.
Conclusion
Taken together, we found that UcOC plays an important role in energy homeostasis via regulation of glucose and lipid metabolism during pregnancy and lactation.







Similar content being viewed by others
References
Zanatta LC, Boguszewski CL, Borba VZ, Kulak CA (2014) Osteocalcin, energy and glucose metabolism. Arq Bras Endocrinol Metabol 58(5):444–451
Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142(2):296–308. https://doi.org/10.1016/j.cell.2010.06.003
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G (2012) Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50(2):568–575. https://doi.org/10.1016/j.bone.2011.04.017
Kacso AC, Bondor CI, Coman AL, Potra AR, Georgescu CE (2016) Determinants of visfatin in type 2 diabetes patients with diabetic kidney disease: Relationship to inflammation, adiposity and undercarboxylated osteocalcin. Scand J Clin Lab Invest 76(3):217–225. https://doi.org/10.3109/00365513.2015.1137349
Neumann T, Lodes S, Kastner B, Franke S, Kiehntopf M, Lehmann T, Muller UA, Wolf G, Samann A (2016) Osteocalcin, adipokines and their associations with glucose metabolism in type 1 diabetes. Bone 82:50–55. https://doi.org/10.1016/j.bone.2015.04.017
Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481(7381):314–320. https://doi.org/10.1038/nature10763
Sanchez-Enriquez S, Ballesteros-Gonzalez IT, Villafan-Bernal JR, Pascoe-Gonzalez S, Rivera-Leon EA, Bastidas-Ramirez BE, Rivas-Carrillo JD, Alcala-Zermeno JL, Armendariz-Borunda J, Llamas-Covarrubias IM, Zepeda-Moreno A (2017) Serum levels of undercarboxylated osteocalcin are related to cardiovascular risk factors in patients with type 2 diabetes mellitus and healthy subjects. World J Diabetes 8(1):11–17. https://doi.org/10.4239/wjd.v8.i1.11
Mayeur S, Wattez JS, Lukaszewski MA, Lecoutre S, Butruille L, Drougard A, Eberle D, Bastide B, Laborie C, Storme L, Knauf C, Vieau D, Breton C, Lesage J (2016) Apelin controls fetal and neonatal glucose homeostasis and is altered by maternal undernutrition. Diabetes 65(3):554–560. https://doi.org/10.2337/db15-0228
Friedman JE (2015) Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man-where do we go next? the 2014 norbert freinkel award lecture. Diabetes Care 38(8):1402–1411. https://doi.org/10.2337/dc15-0628
Srichomkwun P, Houngngam N, Pasatrat S, Tharavanij T, Wattanachanya L, Khovidhunkit W (2016) Undercarboxylated osteocalcin is associated with insulin resistance, but not adiponectin, during pregnancy. Endocrine 53(1):129–135. https://doi.org/10.1007/s12020-015-0829-x
Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, MacDougald O, Baron R, Lotinun S, Karsenty G, Wei W, Ferron M, Kovacs CS, Clemmons D, Wan Y, Rosen CJ (2014) FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology 155(9):3516–3526. https://doi.org/10.1210/en.2014-1083
Morishima Y, Kamisato C, Honda Y, Furugohri T, Shibano T (2013) The effects of warfarin and edoxaban, an oral direct factor Xa inhibitor, on gammacarboxylated (Gla-osteocalcin) and undercarboxylated osteocalcin (uc-osteocalcin) in rats. Thromb Res 131(1):59–63. https://doi.org/10.1016/j.thromres.2012.08.304
Gundberg CM, Lian JB, Booth SL (2012) Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr 3(2):149–157. https://doi.org/10.3945/an.112.001834
Patti A, Gennari L, Merlotti D, Dotta F, Nuti R (2013) Endocrine actions of osteocalcin. Int J Endocrinol 2013:846480. https://doi.org/10.1155/2013/846480
Tsuka S, Aonuma F, Higashi S, Ohsumi T, Nagano K, Mizokami A, Kawakubo-Yasukochi T, Masaki C, Hosokawa R, Hirata M, Takeuchi H (2015) Promotion of insulin-induced glucose uptake in C2C12 myotubes by osteocalcin. Biochem Biophys Res Commun 459(3):437–442. https://doi.org/10.1016/j.bbrc.2015.02.123
Xu B, Lovre D, Mauvais-Jarvis F (2016) Effect of selective estrogen receptor modulators on metabolic homeostasis. Biochimie 124:92–97. https://doi.org/10.1016/j.biochi.2015.06.018
Maertens A, Bouhifd M, Zhao L, Odwin-DaCosta S, Kleensang A, Yager JD, Hartung T (2016) Metabolomic network analysis of estrogen-stimulated MCF-7 cells: a comparison of overrepresentation analysis, quantitative enrichment analysis and pathway analysis versus metabolite network analysis. Arch Toxicol. https://doi.org/10.1007/s00204-016-1695-x
Zhao C, Dahlman-Wright K, Gustafsson JA (2010) Estrogen signaling via estrogen receptor {beta}. J Biol Chem 285(51):39575–39579. https://doi.org/10.1074/jbc.R110.180109
Gu PY, Yu F, Jin S, Yang Q, Su J, Chen Y, Zhao L, Hu SL (2017) Analysis of serum undercarboxylated osteocalcin level in rats with type 2 diabetes mellitus and the correlation with cognitive impairment. Exp Ther Med 14(3):2603–2607. https://doi.org/10.3892/etm.2017.4838
Kulkarni SR, Kumaran K, Rao SR, Chougule SD, Deokar TM, Bhalerao AJ, Solat VA, Bhat DS, Fall CH, Yajnik CS (2013) Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 36(9):2706–2713. https://doi.org/10.2337/dc12-2445
Lappas M (2014) Effect of pre-existing maternal obesity, gestational diabetes and adipokines on the expression of genes involved in lipid metabolism in adipose tissue. Metabolism 63(2):250–262. https://doi.org/10.1016/j.metabol.2013.10.001
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22(1):187–194. https://doi.org/10.1007/s00198-010-1184-7
Kazantzis M (1821) Stahl A (2012) Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 5:852–857. https://doi.org/10.1016/j.bbalip.2011.09.010
Zhan T, Poppelreuther M, Ehehalt R, Fullekrug J (2012) Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS ONE 7(9):e45087. https://doi.org/10.1371/journal.pone.0045087
Neess D, Bek S, Engelsby H, Gallego SF, Faergeman NJ (2015) Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog Lipid Res 59:1–25. https://doi.org/10.1016/j.plipres.2015.04.001
Lee J, Homma T, Kurahashi T, Kang ES, Fujii J (2015) Oxidative stress triggers lipid droplet accumulation in primary cultured hepatocytes by activating fatty acid synthesis. Biochem Biophys Res Commun 464(1):229–235. https://doi.org/10.1016/j.bbrc.2015.06.121
Lee YJ, Kim JW (2017) Monoacylglycerol O-acyltransferase 1 (MGAT1) localizes to the ER and lipid droplets promoting triacylglycerol synthesis. BMB Rep 50(7):367–372. https://doi.org/10.5483/bmbrep.2017.50.7.036
Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, Wojciechowski J, Theussl C, Penninger JM, Lass A, Haemmerle G, Zechner R, Zimmermann R (2011) Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem 286(20):17467–17477. https://doi.org/10.1074/jbc.M110.215434
Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, Consortium NIHMMPC (2010) Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3(9–10):525–534. https://doi.org/10.1242/dmm.006239
Zhu ED, Louis L, Brooks DJ, Bouxsein ML, Demay MB (2014) Effect of bisphosphonates on the rapidly growing male murine skeleton. Endocrinology 155(4):1188–1196. https://doi.org/10.1210/en.2013-1993
Pandey A, Rudraiah M (2015) Analysis of endocrine disruption effect of Roundup((R)) in adrenal gland of male rats. Toxicol Rep 2:1075–1085. https://doi.org/10.1016/j.toxrep.2015.07.021
Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV (2016) Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J Endocrinol Metab 20(6):846–852. https://doi.org/10.4103/2230-8210.192914
Madeira M, Mattar A, Logullo AF, Soares FA, Gebrim LH (2013) Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer 13:425. https://doi.org/10.1186/1471-2407-13-425
Kinon BJ, Liu-Seifert H, Stauffer VL, Jacob J (2013) Bone loss associated with hyperprolactinemia in patients with schizophrenia. Clin Schizophr Relat Psychoses 7(3):115–123. https://doi.org/10.3371/CSRP.KISE.020113
Zampieri TT, Ramos-Lobo AM, Furigo IC, Pedroso JA, Buonfiglio DC, Donato J Jr (2015) SOCS3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol Metab 4(3):237–245. https://doi.org/10.1016/j.molmet.2014.12.005
Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, Hollis BW, Gundberg CM, Stewart AF, Horwitz MJ (2010) Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab 95(4):1767–1776. https://doi.org/10.1210/jc.2009-1518
Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T (2010) Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr 29(6):761–765. https://doi.org/10.1016/j.clnu.2010.02.010
Cauley JA (2015) Estrogen and bone health in men and women. Steroids 99(Pt A):11–15. https://doi.org/10.1016/j.steroids.2014.12.010
Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA (2009) Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297(1):E124–133. https://doi.org/10.1152/ajpendo.00189.2009
Woodside B, Budin R, Wellman MK, Abizaid A (2012) Many mouths to feed: the control of food intake during lactation. Front Neuroendocrinol 33(3):301–314. https://doi.org/10.1016/j.yfrne.2012.09.002
Razny U, Fedak D, Kiec-Wilk B, Goralska J, Gruca A, Zdzienicka A, Kiec-Klimczak M, Solnica B, Hubalewska-Dydejczyk A, Malczewska-Malec M (2017) Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes. Diabetes Metab Res Rev 33:3. https://doi.org/10.1002/dmrr.2862
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg Res 6:30. https://doi.org/10.1186/1749-799X-6-30
Gorres BK, Bomhoff GL, Gupte AA, Geiger PC (2011) Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol 110(4):1046–1053. https://doi.org/10.1152/japplphysiol.00541.2010
Efrimescu CI, Yagoub E, Doyle R (2013) Intentional insulin overdose associated with minimal hypoglycemic symptoms in a non-diabetic patient. Maedica (Buchar) 8(4):365–369
Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF (2015) Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 122(5):643–651. https://doi.org/10.1111/1471-0528.13261
Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T, Kancheva R, Bendlova B (2014) Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol 139:122–129. https://doi.org/10.1016/j.jsbmb.2012.11.007
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, Clemens TL (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142(2):309–319. https://doi.org/10.1016/j.cell.2010.06.002
Rydén M, Arner P (2017) Subcutaneous adipocyte lipolysis contributes to circulating lipid levels. Arterioscler Thromb Vasc Biol 37(9):1782–1787. https://doi.org/10.1161/ATVBAHA.117.309759
Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G (2011) Triglyceride metabolism in pregnancy. Adv Clin Chem 55:133–153. https://doi.org/10.1016/b978-0-12-387042-1.00007-1
Kawakubo-Yasukochi T, Kondo A, Mizokami A, Hayashi Y, Chishaki S, Nakamura S, Takeuchi H, Hirata M (2016) Maternal oral administration of osteocalcin protects offspring from metabolic impairment in adulthood. Obesity 24(4):895–907. https://doi.org/10.1002/oby.21447
Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW (2011) Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS ONE 6(10):e26223. https://doi.org/10.1371/journal.pone.0026223
Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ (2010) Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol 30(21):5009–5020. https://doi.org/10.1128/MCB.00797-10
Acknowledgements
AP and MR were involved in conception, design of experiments and data analysis. AP, MP, and TTC performed experiments. AP, and MR prepared the manuscript. AP, MR, NSA and HRK were involved in manuscript edits. The authors would like to thank Prof. N.G. Kondegowda, Assistant Professor, Department of Translational Research and Cellular Therapeutics, and C.J. Crook, Irell and Manella Graduate School of Biological Sciences and Department of Translational Research and Cellular Therapeutics, City of Hope, CA, for their valuable comments and edits to the manuscript. We thank MR laboratory trainees Ravi Pal, Raza Akhtar and Vijaya Verma for their assistance during experiments. All authors commented on and approved the final version of the manuscript. MR received grant support from the Department of Biotechnology, India (BT/PR21380/AAQ/1/678/2016) and AP received a senior research fellowship from the Council of Scientific and Industrial Research, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures in rats were approved by the Institutional Animal Ethics Committee, Indian Institute of Science (Bangalore, India). This article does not contain any studies with human participants performed by any of the authors.
Informed consent
In this article, no patient care was involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40618_2020_1195_MOESM2_ESM.eps
Supplementary file2 Supplementary Figure 1 Expression analysis of markers for insulin pathway, glycogenesis, and glycolysis in NPNL, pregnant, lactating, and forced wean rats. Soleus muscle tissue was collected during pregnancy, lactation, and after forced weaning for qPCR and western blotting. qPCR expression analysis of insulin pathway genes insulin receptor (A) and Foxo1 (B); insulin sensitivity markers Mcad (C), Nrf1 (D), and Pparα (E); glucose metabolism genes glycogenesis marker Gys1 (F) and gluconeogenesis marker Pck (G); and glucose transporter Glut4 (H) in muscle of NPNL, pregnant, lactating, and forced wean rats. Immunoblot analysis of insulin pathway molecules pPI3K (I), pPTEN (J), pPDK1 (K), pAKT/AKT (L), glycogenesis regulatory molecule pGSK3β (M) and glycolysis marker GAPDH (N) was performed. A representative immunoblot is presented in (O). β-tubulin was used as a loading control. Densitometric values are presented as mean ± SEM (n= 3 per group). NPNL analysis results were as set as 1-fold; analysis of other groups is expressed in relation to 1-fold and presented as mean ± SEM (n= 4 per group). Statistical significance was calculated by one-way ANOVA. *, **, and *** represent significantly different by p<0.05, 0.01, and 0.001. NPNL: nonpregnant nonlactating; P6, 12, and 19: day 6, 12, and 19 of pregnancy; L3, 6, 11, and 20: day 3, 6, 11, and 20 of lactation; F6: day 6 of forced weaning. (EPS 171 kb)
40618_2020_1195_MOESM3_ESM.eps
Supplementary file3 Supplementary Figure 2 qPCR expression analysis of insulin pathway genes in liver of NPNL, pregnant, lactating, and forced wean rats. qPCR expression analysis of insulin receptor (A), Foxo1 (B), insulin sensitivity marker Mcad (C), glycogenesis marker Gys2 (D), glycolysis markers Gck2 (E) and Pfk1 (F), gluconeogenesis marker Pck (G), glucose transporter Glut2 (H). NPNL analysis results were set as 1-fold and expression changes in other groups expressed in relation to 1-fold change of NPNL; results were presented as mean ± SEM (n=4 per group). Immunoblot analysis of insulin pathway molecules pPI3K (I), pPTEN (J), pPDK1 (K), pAKT/AKT (L), glycogenesis regulatory molecule pGSK3β (M) and glycolysis marker GAPDH (N) in liver of NPNL, pregnant, lactating, and forced wean rats. β-actin was used as the loading control. The densitometric value of each protein is represented as mean ± SEM (n= 3 per group). Statistical significance was calculated by one-way ANOVA. * and ** represent significant differences between groups by p<0.05 and 0.01, respectively. NPNL: nonpregnant nonlactating; P6, 12, and 19: day 6, 12, and 19 of pregnancy: L3, 6, 11, and 20: day 3, 6, 11, and 20 of lactation; F6: day 6 of forced weaning. (EPS 210 kb)
40618_2020_1195_MOESM4_ESM.eps
Supplementary file4 Supplementary Figure 3. qPCR expression of insulin pathway markers insulin receptor (A) and Foxo1 (B), insulin sensitivity marker Mcad (C), glycolysis marker Gck2 (D), and glucose transporter Glut4 (E) from GWAT RNA of NPNL, pregnant, lactating, and forced wean rats. Gene expression from NPNL rats was set as 1-fold; analysis of other groups is expressed in relation to 1-fold and presented as mean ± SEM (n=4 per group). Statistical significance was calculated by one-way ANOVA. *, **, and *** represent significant differences by p<0.05, 0.01, and 0.001, respectively. GWAT: gonadal white adipose tissue; NPNL: nonpregnant nonlactating; P6, 12, and 19: day 6, 12, and 19 of pregnancy; L3, 6, 11, and 20: day 3, 6, 11, and 20 of lactation: F6: day 6 of forced weaning. (EPS 39 kb)
40618_2020_1195_MOESM5_ESM.eps
Supplementary file5 Supplementary Figure 4. Serum OC and CTx levels and histological changes in mammary glands of rats treated with either WF or ALN. Serum OC and CTx levels in WF-treated NPNL (A, E), WF-treated pregnant (B, F), WF-treated lactating (C, G), and ALN-treated lactating rats (D, H). Data are presented as mean ± SEM (n = 5 per group). Statistical significance was calculated by one-way ANOVA (A, D, E, H) and t-test (B, C, F, G). *, **, and *** represent significant differences by p<0.05, 0.01, and 0.01, respectively. Mammary gland sections from lactating, WF-treated (0.25 mg/kg BW), and ALN-treated (200 µg/kg BW) rats were stained with hematoxylin and eosin; images are presented at 40X magnification. Beneath epithelial tissue (dark purple), white fat depots can be seen. The red arrows indicate secretory parenchymal areas. OC: Osteocalcin; NPNL: nonpregnant nonlactating; VEH: vehicle; P6: day 6 of pregnancy receiving vehicle or 0.25 mg/kg BW/d WF treatment for three consecutive days; L6: day 6 of lactation receiving vehicle and ALN treatment for six weeks. (EPS 871 kb)
40618_2020_1195_MOESM6_ESM.eps
Supplementary file6 Supplementary Figure 5. Effects of HFD during lactation. Rats were maintained on regular laboratory chow diet or high-fat diet (ND and HFD, respectively) as described in Materials and Methods. Bodyweight of rats were monitored during lactation (A). Rats (n=3) were euthanized on day 6 of lactation. GWAT was collected and weighed (B). Blood was collected and serum analyzed for triglyceride levels (C). Liver sections were collected and stained with hematoxylin and eosin. Images are presented at 100, 200, and 400X magnification (D). Black arrows indicate lipid droplets; red arrows indicate infiltrated blood cells. H&E sections are representative of tissue sections from three rats. One group of rats was maintained during lactation and litter size (E) was monitored. Values are presented as mean ± SEM (n= 3). Statistical significance between the two groups was determined by two-way ANOVA (A, F) and t-test (B, C, E). * and ** represent significant differences by p<0.05 and 0.01, respectively. L6: day 6 of lactation; GWAT: gonadal white adipose tissue; TG: triglyceride; ND: regular laboratory chow diet; HFD: high-fat diet; L6: day 6 of lactation. (EPS 1767 kb)
40618_2020_1195_MOESM7_ESM.eps
Supplementary file7 Supplementary Figure 6. Gene expression analysis of lipid metabolism in adipose tissue of nonpregnant rats treated with or without ALN and WF. Adipose tissue was collected from both groups and total RNA was converted into cDNA. qPCR expression analysis was performed to examine expression of lipogenesis markers such as Lpl (A), Fatp1 (B), Fatp4 (C), Fabp (D), Acbp (E), Fasn (F), Mgat (G), and Dgat1 (H), and lipolysis enzymes such as Atgl (I), Hsl (J), and Mgll (K). Values are mean ± SEM (n= 4). Statistical significance between the two groups was determined by one-way ANOVA. *, **, and *** represent significant differences compared to NPNL by p<0.05, 0.01 and 0.001. ALN: alendronate; WF: warfarin; NPNL: nonpregnant nonlactating. (EPS 58 kb)
Rights and permissions
About this article
Cite this article
Pandey, A., Khan, H.R., Alex, N.S. et al. Under-carboxylated osteocalcin regulates glucose and lipid metabolism during pregnancy and lactation in rats. J Endocrinol Invest 43, 1081–1095 (2020). https://doi.org/10.1007/s40618-020-01195-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01195-8