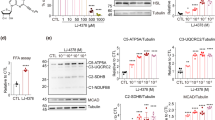
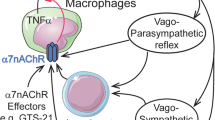
Abstract
Purpose
The alpha7 nicotinic acetylcholine receptor (α7nAChR), involved in the modulation of inflammation and insulin sensitivity, is downregulated in white adipose tissue (WAT) of obese patients. This study aims to test the ability of a selective synthetic α7nAChR agonist, the spirocyclic Δ2-isoxazoline derivative (R)-(−)-ICH3 (ICH3), to counteract acute inflammation and obesity-associated modifications in WAT.
Methods
We employed the LPS-septic shock murine model, human primary adipocytes and diet-induced obese (DIO) mice. Inflammatory factor expression was assessed by ELISA and quantitative real-time PCR. Flow cytometry was employed to define WAT inflammatory infiltrate. Insulin signaling was monitored by quantification of AKT phosphorylation.
Results
In the septic shock model, ICH3 revealed antipyretic action and reduced the surge of circulating cytokines. In vitro, ICH3 stimulation (10 µM) preserved viability of human adipocytes, decreased IL-6 mRNA (P < 0.05) and blunted LPS-induced peak of TNFα (P < 0.05) and IL-6 (P < 0.01). Chronic administration of ICH3 to DIO mice was associated with lower numbers of CD8+ T cells (P < 0.05) and to changed WAT expression of inflammatory factors (Hp, P < 0.05; CD301/MGL1, P < 0.01; Arg-1, P < 0.05). As compared to untreated, ICH3 DIO mice exhibited improved insulin signaling in the skeletal muscle (P < 0.01) mirrored by an improved response to glucose load (ipGTT: P < 0.05 at 120 min).
Conclusions
We proved that ICH3 is an anti-inflammatory drug, able to reduce inflammatory cytokines in human adipocytes and to blunt the effects of obesity on WAT inflammatory profile, on glucose tolerance and on tissue insulin sensitivity.






Similar content being viewed by others
References
Beckmann J, Lips KS (2013) The non-neuronal cholinergic system in health and disease. Pharmacology 92(5–6):286–302. https://doi.org/10.1159/000355835
de Jonge WJ, Ulloa L (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151(7):915–929. https://doi.org/10.1038/sj.bjp.0707264
Sharma G, Vijayaraghavan S (2008) Nicotinic receptors containing the alpha7 subunit: a model for rational drug design. Curr Med Chem 15(28):2921–2932
Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Investig 117(2):289–296. https://doi.org/10.1172/JCI30555
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421(6921):384–388. https://doi.org/10.1038/nature01339
Bray GA (2006) Obesity: the disease. J Med Chem 49(14):4001–4007. https://doi.org/10.1021/jm0680124
Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, Lopez-Soriano J, Centoni R, Ciccarone AM, Benzi L, Vitti P, Del Prato S, Pinchera A, Maffei M (2004) Serum haptoglobin: a novel marker of adiposity in humans. J Clin Endocrinol Metab 89(6):2678–2683. https://doi.org/10.1210/jc.2003-031965
Giovannini S, Onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F (2011) Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc 59(9):1679–1685. https://doi.org/10.1111/j.1532-5415.2011.03570.x
Maffei M, Barone I, Scabia G, Santini F (2016) The multifaceted haptoglobin in the context of adipose tissue and metabolism. Endocr Rev 37(4):403–416. https://doi.org/10.1210/er.2016-1009
Cancello R, Zulian A, Maestrini S, Mencarelli M, Della Barba A, Invitti C, Liuzzi A, Di Blasio AM (2012) The nicotinic acetylcholine receptor alpha7 in subcutaneous mature adipocytes: downregulation in human obesity and modulation by diet-induced weight loss. Int J Obes (Lond) 36(12):1552–1557. https://doi.org/10.1038/ijo.2011.275
Dallanoce C, Frigerio F, Martelli G, Grazioso G, Matera C, Pome DY, Pucci L, Clementi F, Gotti C, De Amici M (2010) Novel tricyclic Delta(2)-isoxazoline and 3-oxo-2-methyl-isoxazolidine derivatives: synthesis and binding affinity at neuronal nicotinic acetylcholine receptor subtypes. Bioorg Med Chem 18(12):4498–4508. https://doi.org/10.1016/j.bmc.2010.04.065
Dallanoce C, Matera C, Pucci L, Gotti C, Clementi F, Amici MD, Micheli CD (2012) Synthesis and binding affinity at alpha4beta2 and alpha7 nicotinic acetylcholine receptors of new analogs of epibatidine and epiboxidine containing the 7-azabicyclo[2.2.1]hept-2-ene ring system. Bioorg Med Chem Lett 22(2):829–832. doi: 10.1016/j.bmcl.2011.12.052
Grazioso G, Pome DY, Matera C, Frigerio F, Pucci L, Gotti C, Dallanoce C, De Amici M (2009) Design of novel alpha7-subtype-preferring nicotinic acetylcholine receptor agonists: application of docking and MM-PBSA computational approaches, synthetic and pharmacological studies. Bioorg Med Chem Lett 19(22):6353–6357. https://doi.org/10.1016/j.bmcl.2009.09.073
Matera C, Quadri M, Sciaccaluga M, Pome DY, Fasoli F, De Amici M, Fucile S, Gotti C, Dallanoce C, Grazioso G (2016) Modification of the anabaseine pyridine nucleus allows achieving binding and functional selectivity for the alpha3beta4 nicotinic acetylcholine receptor subtype. Eur J Med Chem 108:392–405. https://doi.org/10.1016/j.ejmech.2015.11.045
Quadri M, Matera C, Silnovic A, Pismataro MC, Horenstein NA, Stokes C, Papke RL, Dallanoce C (2017) Identification of alpha7 nicotinic acetylcholine receptor silent agonists based on the spirocyclic quinuclidine-delta(2)-isoxazoline scaffold: synthesis and electrophysiological evaluation. ChemMedChem 12(16):1335–1348. https://doi.org/10.1002/cmdc.201700162
Dallanoce C, Magrone P, Matera C, Frigerio F, Grazioso G, De Amici M, Fucile S, Piccari V, Frydenvang K, Pucci L, Gotti C, Clementi F, De Micheli C (2011) Design, synthesis, and pharmacological characterization of novel spirocyclic quinuclidinyl-Delta2-isoxazoline derivatives as potent and selective agonists of alpha7 nicotinic acetylcholine receptors. ChemMedChem 6(5):889–903. https://doi.org/10.1002/cmdc.201000514
Matera C, Dondio G, Braida D, Ponzoni L, De Amici M, Sala M, Dallanoce C (2018) In vivo and in vitro ADMET profiling and in vivo pharmacodynamic investigations of a selective alpha7 nicotinic acetylcholine receptor agonist with a spirocyclic Delta(2)-isoxazoline molecular skeleton. Eur J Pharmacol 820:265–273. https://doi.org/10.1016/j.ejphar.2017.12.047
Di Cesare ML, Pacini A, Matera C, Zanardelli M, Mello T, De Amici M, Dallanoce C, Ghelardini C (2014) Involvement of alpha7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology 79:37–48. https://doi.org/10.1016/j.neuropharm.2013.10.034
Cerri C, Genovesi S, Allegra M, Pistillo F, Puntener U, Guglielmotti A, Perry VH, Bozzi Y, Caleo M (2016) The chemokine CCL2 mediates the seizure-enhancing effects of systemic inflammation. J Neurosci 36(13):3777–3788. https://doi.org/10.1523/JNEUROSCI.0451-15.2016
Hagberg CE, Li Q, Kutschke M, Bhowmick D, Kiss E, Shabalina IG, Harms MJ, Shilkova O, Kozina V, Nedergaard J, Boucher J, Thorell A, Spalding KL (2018) Flow cytometry of mouse and human adipocytes for the analysis of browning and cellular heterogeneity. Cell Rep 24(10):2746–2756 e2745. doi: 10.1016/j.celrep.2018.08.006
Berger S, Ceccarini G, Scabia G, Barone I, Pelosini C, Ferrari F, Magno S, Dattilo A, Chiovato L, Vitti P, Santini F, Maffei M (2017) Lipodystrophy and obesity are associated with decreased number of T cells with regulatory function and pro-inflammatory macrophage phenotype. Int J Obes (Lond) 41(11):1676–1684. https://doi.org/10.1038/ijo.2017.163
Lisi S, Gamucci O, Vottari T, Scabia G, Funicello M, Marchi M, Galli G, Arisi I, Brandi R, D'Onofrio M, Pinchera A, Santini F, Maffei M (2011) Obesity-associated hepatosteatosis and impairment of glucose homeostasis are attenuated by haptoglobin deficiency. Diabetes 60(10):2496–2505. https://doi.org/10.2337/db10-1536
Lawrence CB, Brough D, Knight EM (2012) Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Dis Models Mech 5(5):649–659. https://doi.org/10.1242/dmm.009068
Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T (2012) Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia 55(10):2583–2592. https://doi.org/10.1007/s00125-012-2607-0
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15(8):914–920. https://doi.org/10.1038/nm.1964
Travers RL, Motta AC, Betts JA, Bouloumie A, Thompson D (2015) The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes (Lond) 39(5):762–769. https://doi.org/10.1038/ijo.2014.195
Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M (2010) An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Therap 332(1):173–180. https://doi.org/10.1124/jpet.109.154633
McFadden KL, Cornier MA, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol 5:553. https://doi.org/10.3389/fpsyg.2014.00553
Mirrasekhian E, Nilsson JLA, Shionoya K, Blomgren A, Zygmunt PM, Engblom D, Hogestatt ED, Blomqvist A (2018) The antipyretic effect of paracetamol occurs independent of transient receptor potential ankyrin 1-mediated hypothermia and is associated with prostaglandin inhibition in the brain. FASEB J 32(10):5751–5759. https://doi.org/10.1096/fj.201800272R
Pinheiro NM, Santana FP, Almeida RR, Guerreiro M, Martins MA, Caperuto LC, Camara NO, Wensing LA, Prado VF, Tiberio IF, Prado MA, Prado CM (2017) Acute lung injury is reduced by the alpha7nAChR agonist PNU-282987 through changes in the macrophage profile. FASEB J 31(1):320–332. https://doi.org/10.1096/fj.201600431R
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405(6785):458–462. https://doi.org/10.1038/35013070
Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN (2005) Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 201(7):1113–1123. https://doi.org/10.1084/jem.20040463
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29(24):2959–2971. https://doi.org/10.1093/eurheartj/ehn387
Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA (2013) Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol 2013:678159. https://doi.org/10.1155/2013/678159
Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, Parsons PE, Dixon AE, Wargo MJ, Littenberg B, Wouters EF, Suratt BT (2016) Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 1(8). doi: 10.1172/jci.insight.82101
Huang CJ, Stewart JK, Shibata Y, Slusher AL, Acevedo EO (2015) Lipopolysaccharide-binding protein and leptin are associated with stress-induced interleukin-6 cytokine expression ex vivo in obesity. Psychophysiology 52(5):687–694. https://doi.org/10.1111/psyp.12387
Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr (2001) Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol 281(1):R115–132. https://doi.org/10.1152/ajpregu.2001.281.1.R115
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091):87–91
Sharma K, McCue P, Dunn SR (2003) Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 284(6):F1138–1144. https://doi.org/10.1152/ajprenal.00315.2002
Aydin S, Aksoy A, Aydin S, Kalayci M, Yilmaz M, Kuloglu T, Citil C, Catak Z (2014) Today's and yesterday's of pathophysiology: biochemistry of metabolic syndrome and animal models. Nutrition 30(1):1–9. https://doi.org/10.1016/j.nut.2013.05.013
Panchal SK, Brown L (2011) Rodent models for metabolic syndrome research. J Biomed Biotechnol 2011:351982. https://doi.org/10.1155/2011/351982
Masoodi M, Kuda O, Rossmeisl M, Flachs P (1851) Kopecky J (2015) Lipid signaling in adipose tissue: connecting inflammation and metabolism. Biochem Biophys Acta 4:503–518. https://doi.org/10.1016/j.bbalip.2014.09.023
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489. https://doi.org/10.1146/annurev-immunol-030409-101212
do Nascimento CO, Hunter L, Trayhurn P (2004) Regulation of haptoglobin gene expression in 3T3-L1 adipocytes by cytokines, catecholamines, and PPARgamma. Biochem Biophys Res Commun 313(3):702–708. https://doi.org/10.1016/j.bbrc.2003.12.008
Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, Viegi A, Ciampi O, Bardi G, Vitti P, Pinchera A, Santini F (2009) The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2). BMC Biol 7:87. https://doi.org/10.1186/1741-7007-7-87
Lamacchia C, Palmer G, Bischoff L, Rodriguez E, Talabot-Ayer D, Gabay C (2010) Distinct roles of hepatocyte- and myeloid cell-derived IL-1 receptor antagonist during endotoxemia and sterile inflammation in mice. J Immunol 185(4):2516–2524. https://doi.org/10.4049/jimmunol.1000872
Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB (2011) Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60(10):2474–2483. https://doi.org/10.2337/db11-0194
Chung JH, Moon J, Lee YS, Chung HK, Lee SM, Shin MJ (2014) Arginase inhibition restores endothelial function in diet-induced obesity. Biochem Biophys Res Commun 451(2):179–183. https://doi.org/10.1016/j.bbrc.2014.07.083
Hu H, Moon J, Chung JH, Kim OY, Yu R, Shin MJ (2015) Arginase inhibition ameliorates adipose tissue inflammation in mice with diet-induced obesity. Biochem Biophys Res Commun 464(3):840–847. https://doi.org/10.1016/j.bbrc.2015.07.048
Moon J, Do HJ, Cho Y, Shin MJ (2014) Arginase inhibition ameliorates hepatic metabolic abnormalities in obese mice. PLoS ONE 9(7):e103048. https://doi.org/10.1371/journal.pone.0103048
Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN (2009) MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206(13):3143–3156. https://doi.org/10.1084/jem.20091333
Acknowledgements
This research has received funding from the European Community's Seventh Framework Programme [FP7/2007–2013] under Grant agreement no. 291778 (DTI-IMPORT).
Author information
Authors and Affiliations
Contributions
GS, RC, SB, AD, AZ, IB, and GC performed the experiments in vitro and in vivo; CD and CM synthesized the compound, GS, SB, and FS analyzed the data, GS, RC, SB, MDA, ADB, and MM conceived the experimental design. GS, RC, and MM wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Ethical approval
This study was approved by the Ethical Committee of IRCCS-Istituto Auxologico Italiano, and signed informed consent was obtained for adipose tissue sampling during surgery. All animal care protocols and procedures were approved by the Italian Ministry of Health (protocol 108/2015-PR) and all experiments performed in accordance with relevant guidelines and regulations.
Informed consent
All participants provided their informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scabia, G., Cancello, R., Dallanoce, C. et al. ICH3, a selective alpha7 nicotinic acetylcholine receptor agonist, modulates adipocyte inflammation associated with obesity. J Endocrinol Invest 43, 983–993 (2020). https://doi.org/10.1007/s40618-020-01182-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01182-z