Abstract
Purpose
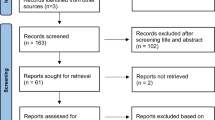
Hypophosphatemia (HP) can be observed in patients evaluated for skeletal fragility. We investigated prevalence of HP among outpatients referred for low bone density or fragility fractures, HP-associated clinical and biochemical features and outcomes of recommended diagnostic algorithm in our cohort.
Methods
Chronic HP (phosphate ≤ 2.7 mg/dL over 6 months or longer) was retrospectively investigated among 2319 patients. In renal wasting-related HP, intact FGF23 was assessed; non-suppressed FGF23 prompted the performance of 68Ga-DOTATOC PET/CT in the suspicion of tumor-induced steomalacia (TIO).
Results
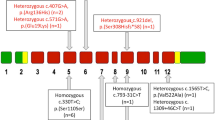
Renal wasting-related HP (median 2.2, range 1.6–2.6 mg/dL) was observed in 19 patients (0.82%). FGF23 levels were suppressed in two patients diagnosed with renal tubular disease, increased in one and within normal range in most patients. X-linked hypophosphatemic rickets was diagnosed in one woman. In the remaining 16 patients, highly prevalent fragility fractures (50%) and severely reduced bone mineral density were detected, though diagnostic criteria for osteomalacia were not fulfilled. 68Ga-PET was performed in nine patients and was positive in four. While intact FGF23 levels alone failed to differentiate PET’s outcomes (positive: FGF23 median 70.5 pg/mL; negative: 52 pg/mL, P = 0.462), the coexistence of multiple biochemical and radiologic alterations performed better in prediction of PET’s positivity.
Conclusion
Mild, apparently unexplained HP is observed in 0.82% of patients with low bone density or fragility fractures. In asymptomatic patients with isolated mild hypophosphatemia, the probability of finding an underlying tumor disease is very low, and utility of extensive and expensive diagnostic workup should be carefully considered in this setting.


Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Christov M, Jüppner H (2018) Phosphate homeostasis disorders. Best Pract Res Clin Endocrinol Metab 32:685–706. https://doi.org/10.1016/j.beem.2018.06.004
Marcucci G, Masi L, Ferrarì S, Haffner D (2018) Phosphate wasting disorders in adults. Osteoporos Int 23:2369–2387. https://doi.org/10.1007/s00198-018-4618-2
Khalil R, Kim NR, Jardi F, Vanderschueren D, Claessens F, Decallonne B (2018) Sex steroids and the kidney: role in renal calcium and phosphate handling. Mol Cell Endocrinol 465:61–72. https://doi.org/10.1016/j.mce.2017.11.011
Cannata-Andía JB, Carrillo-López N, Naves-Díaz M (2010) Estrogens and bone disease in chronic kidney disease: role of FGF23. Curr Opin Nephrol Hypertens 19:21–25. https://doi.org/10.1097/MNH.0b013e328338f508
Carrillo-López N, Román-García P, Rodríguez-Rebollar A, Fernández-Martín JL, Naves-Díaz M, Cannata-Andía JB (2009) Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol 20:2009–2017. https://doi.org/10.1681/ASN.2008121258
Weisinger JR, Bellorín-Font E (1998) Electrolyte quintet magnesium and phosphorus. Lancet 352:391–396
Gaasbeek A, Meinders AE (2005) Hypophosphatemia: an update on its etiology and treatment. Am J Med 118:1094–1101
Laroche M, Boyer JF, Jahafar H, Allard J, Tack I (2009) Normal FGF23 levels in adult idiopathic phosphate diabetes. Calcif Tissue Int 84:112–117. https://doi.org/10.1007/s00223-008-9204-8
Bech AP, Hoorn EJ, Zietse R, Wetzels JFM, Nijenhuis T (2018) Yield of diagnostic tests in unexplained renal hypophosphatemia: a case series. BMC Nephrol 19:220. https://doi.org/10.1186/s12882-018-1017-z
Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH (2005) Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911. https://doi.org/10.1359/JBMR.050714
Anastasilakis AD, Polyzos SA, Goulis DG, Slavakis A, Efstathiadou Z, Kita M, Koukoulis G, Avramidis A (2008) Endogenous intact PTH is suppressed during teriparatide (rhPTH 1-34) administration in postmenopausal women with established osteoporosis. Endocr J 55:613–616
Palmerini E, Chawla NS, Ferrari S, Sudan M, Picci P, Marchesi E, Leopardi MP, Syed I, Sankhala KK, Parthasarathy P, Mendanha WE, Pierini M, Paioli A, Chawla SP (2017) Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer 76:118–124. https://doi.org/10.1016/j.ejca.2017.01.028
Watkins KR, Rogers JE, Atkinson B (2015) Tolerability of denosumab in metastatic solid tumor patients with renal insufficiency. Support Care Cancer 23:1657–1662. https://doi.org/10.1007/s00520-014-2521-8
Stuss M, Sewerynek E, Król I, Stępień-Kłos W, Jędrzejczyk S (2016) Assessment of OPG, RANKL, bone turnover markers serum levels, and BMD after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis. Endokrynol Pol 67:174–184. https://doi.org/10.5603/EP.a2016.0014
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S (2008) International society for clinical densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121. https://doi.org/10.1016/j.bone.2008.08.106
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
D’Oronzo S, Stucci S, Tucci M, Silvestris F (2015) Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev 41:798–808. https://doi.org/10.1016/j.ctrv.2015.09.003
Fukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, Takeuchi Y, Matsumoto T (2015) Pathogenesis and diagnostic criteria for rickets and osteomalacia-proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J Bone Miner Metab 33:467–473. https://doi.org/10.1507/endocrj.EJ15-0289
Day AL, Morgan SL, Saag KG (2018) Hypophosphatemia in the setting of metabolic bone disease: case reports and diagnostic algorithm. Ther Adv Musculoskelet Dis 10:151–156. https://doi.org/10.1177/1759720X18779761
Imel EA, Econs MJ (2012) Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 97:696–706. https://doi.org/10.1210/jc.2011-1319
Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2:309–310
Souberbielle JC, Prié D, Piketty ML, Rothenbuhler A, Delanaye P, Chanson P, Cavalier E (2017) Evaluation of a new fully automated assay for plasma intact FGF23. Calcif Tissue Int 101:510–518. https://doi.org/10.1007/s00223-017-0307-y
Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN (2003) Human gene mutation database (HGMD): 2003 update. Hum Mutat 21:577–581. https://doi.org/10.1002/humu.10212
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D (2010) Mutation Taster evaluates disease-causing potential of sequence alterations. NatMethods 7:575–576. https://doi.org/10.1038/nmeth0810-575
Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouzé P, Brunak S (1996) Splice site prediction in Arabidopsis thaliana DNA by combining local and global sequence information. Nucleic Acids Res 24:3439–3452. https://doi.org/10.1093/nar/24.17.3439
Rodrat M, Wongdee K, Panupinthu N, Thongbunchoo J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N (2017) Prolonged exposure to 1,25(OH)2D3 and high ionized calcium induces FGF-23 production in intestinal epithelium-like Caco-2 monolayer: a local negative feedback for preventing excessive calcium transport. Arch Biochem Biophys 640:10–16. https://doi.org/10.1016/j.abb.2017.12.022
Mirams M, Robinson BG, Mason RS, Nelson AE (2004) Bone as a source of FGF23: regulation by phosphate? Bone 35:1192–1199. https://doi.org/10.1016/j.bone.2004.06.014
Liamis G, Milionis HJ, Elisaf M (2010) Medication-induced hypophosphatemia: a review. QJM 103:449–459. https://doi.org/10.1093/qjmed/hcq039
Feng J, Jiang Y, Wang O, Li M, Xing X, Huo L, Li F, Yu W, Zhong DR, Jin J, Liu Y, Qi F, Lv W, Zhou L, Meng XW, Xia WB (2017) The diagnostic dilemma of tumor induced osteomalacia: a retrospective analysis of 144 cases. Endocr J 64:675–683. https://doi.org/10.1507/endocrj.EJ16-0587
Yu WJ, He JW, Fu WZ, Wang C, Zhang ZL (2016) Reports of 17 Chinese patients with tumor-induced osteomalacia. J Bone Miner Metab 35:298–307. https://doi.org/10.1007/s00774-016-0756-9
González G, Baudrand R, Sepúlveda MF, Vucetich N, Guarda FJ, Villanueva P, Contreras O, Villa A, Salech F, Toro L, Michea L, Florenzano P (2017) Tumor-induced osteomalacia: experience from a South American academic center. Osteoporos Int 28:2187–2193. https://doi.org/10.1007/s00198-017-4007-2
Paquet M, Gauthé M, Zhang Yin J, Nataf V, Bélissant O, Orcel P, Roux C, Talbot JN, Montravers F (2018) Diagnostic performance and impact on patient management of 68 Ga-DOTA-TOC PET/CT for detecting osteomalacia-associated tumours. Eur J Nucl Med Mol Imaging 45:1710–1720. https://doi.org/10.1007/s00259-018-3971-x
Breer S, Brunkhorst T, Beil FT, Peldschus K, Heiland M, Klutmann S, Barvencik F, Zustin J, Gratz KF, Amling M (2014) 68 Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone 64:222–227. https://doi.org/10.1016/j.bone.2014.04.016
Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, Bhansali A (2015) Comparison of 18F-FDG and 68 Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med 40:6–10. https://doi.org/10.1097/RLU.0000000000000460
Martini G, Valleggi F, Gennari L, Merlotti D, De Paola V, Valenti R, Nuti R (2012) Oncogenic osteomalacia. Clin Cases Miner Bone Metab 3:76–83
Amblee A, Uy J, Senseng C, Hart P (2014) Tumor-induced osteomalacia with normal systemic fibroblast growth factor-23 level. Clin Kidney J 7:186–189. https://doi.org/10.1093/ckj/sfu004
Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T, Matsumoto T (2008) Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients. Bone 42:1235–1239. https://doi.org/10.1016/j.bone.2008.02.014
Laroche M (2001) Phosphate, the renal tubule, and the musculoskeletal system. Joint Bone Spine 68:211–215
Burnett-Bowie SM, Mendoza N, Leder BZ (2007) Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone 40:913–918. https://doi.org/10.1016/j.bone.2006.10.016
Zhang D, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K (2013) Effects of sex and postmenopausal estrogen use on serum phosphorus levels: a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Kidney Dis 63:198–205. https://doi.org/10.1053/j.ajkd.2013.07.012
Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN, Tracy R, Laughlin GA, Criqui MH, Budoff MJ, Li D, Ix JH (2013) Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab 98:4890–4898. https://doi.org/10.1210/jc.2013-2286
Rayamajhi SJ, Yeh R, Wong T, Dumeer S, Mittal BR, Remotti F, Chikeka I, Reddy AK (2019) Tumor-induced osteomalacia—current imaging modalities and a systematic approach for tumor localization. Clin Imaging 56:114–123. https://doi.org/10.1016/j.clinimag.2019.04.007
Funding
The study was partially supported by Italian Ministry of Health (L4126) and by Gruppo San Donato Foundation (Progetto 5x1000 2016 “Osteoregistry”).
Author information
Authors and Affiliations
Contributions
SC designed the study and performed the critical revision of the manuscript. She is guarantor. RI was responsible for data collection, statistical analysis and manuscript preparation. GG, ML and SC performed the clinical diagnoses and patients’ follow-up. MM was responsible for histopathological examination of tissue samples. SN, KM and SM performed molecular analyses. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Indirli, R., Guabello, G., Longhi, M. et al. FGF23-related hypophosphatemia in patients with low bone mineral density and fragility fractures: challenges in diagnosis and management. J Endocrinol Invest 43, 787–798 (2020). https://doi.org/10.1007/s40618-019-01165-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01165-9