Abstract
Purpose
Type 2 diabetes frequently remains undiagnosed for years, whereas early detection of affected individuals would facilitate the implementation of timely and cost-effective therapies, hence decreasing morbidity. With the intention of identifying novel diagnostic biomarkers, we characterized the miRNA profile of microvesicles isolated from retroactive serum samples of normoglycemic individuals and two groups of subjects with prediabetes that in the following 4 years either progressed to overt diabetes or remained stable.
Methods
We profiled miRNAs in serum microvesicles of a selected group of control and prediabetic individuals participating in the PREDAPS cohort study. Half of the subjects with prediabetes were diagnosed with diabetes during the 4 years of follow-up, while the glycemic status of the other half remained unchanged.
Results
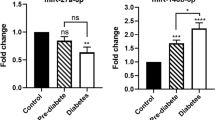
We identified two miRNAs, miR-10b and miR-223-3p, which target components of the insulin signaling pathway and whose ratio discriminates between these two subgroups of prediabetic individuals at a stage at which other features, including glycemia, are less proficient at separating them. In global, the profile of miRNAs in microvesicles of prediabetic subjects primed to progress to overt diabetes was more similar to that of diabetic patients than the profile of prediabetic subjects who did not progress.
Conclusion
We have identified a miRNA signature in serum microvesicles that can be used as a new screening biomarker to identify subjects with prediabetes at high risk of developing diabetes, hence allowing the implementation of earlier, and probably more effective, therapeutic interventions.



Similar content being viewed by others
References
Cho NH, Shaw JE, Karuranga S et al (2018) IDF diabetes atlas. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
Soriguer F, Goday A, Bosch-Comas A et al (2012) Prevalence of diabetes mellitus and impaired glucose regulation in Spain: Di@bet.es study. Diabetologia 55:88–93. https://doi.org/10.1007/s00125-011-2336-9
Mata-Cases M, Casajuana M, Franch-Nadal J et al (2016) Direct medical costs attributable to type 2 diabetes: a population-based study in Catalonia, Spain. Eur J Health Econ 17:1001–1010. https://doi.org/10.1007/s10198-015-0742-5
Müller G (2012) Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab Syndr Obes Targets Ther 5:247–282. https://doi.org/10.2147/DMSO.S32923
Pirola L, Balcerczyk A, Okabe J, El-Osta A (2010) Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol 6:665–675. https://doi.org/10.1038/nrendo.2010.188
Párrizas M, Novials A (2016) Circulating microRNAs as biomarkers for metabolic disease. Best Pract Res Clin Endocrinol Metab 30:591–601. https://doi.org/10.1016/j.beem.2016.08.001
Bartel DP (2018) Metazoan microRNAs. Cell 173:20–51
Castaño C, Novials A, Párrizas M (2019) Exosomes and diabetes. Diabetes Metab Res Rev 2019:e3107. https://doi.org/10.1002/dmrr.3107
Párrizas M, Brugnara L, Esteban Y et al (2015) Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab 100:E407–E415. https://doi.org/10.1210/jc.2014-2574
Thomou T, Mori MA, Dreyfuss JM et al (2017) Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542:450–455. https://doi.org/10.1038/nature21365
Ying W, Riopel M, Bandyopadhyay G et al (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171:372–384. https://doi.org/10.1016/j.cell.2017.08.035
Castaño C, Kalko S, Novials A, Párrizas M (2018) Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci 115:12158–12163. https://doi.org/10.1073/pnas.1808855115
Diaz-Redondo A, Giraldez-Garcia C, Carrillo L et al (2015) Modifiable risk factors associated with prediabetes in men and women: cross-sectional analysis of the cohort study in primary health care on the evolution of patients with prediabetes (PREDAPS). BMC Fam Pract 16:5. https://doi.org/10.1186/s12875-014-0216-3
Franch-Nadal J, Caballeria L, Mata-Cases M et al (2018) Fatty liver index is a predictor of incident diabetes in patients with prediabetes: PREDAPS study. PLoS One 13:1–17. https://doi.org/10.1371/journal.pone.0198327
Giráldez-García C, Franch-Nadal J, Sangrós FJ et al (2018) Adiposity and diabetes risk in adults with prediabetes: heterogeneity of findings depending on age and anthropometric measure. Obesity 26:1481–1490. https://doi.org/10.1002/oby.22256
Bedogni G, Bellentani S, Miglioli L et al (2006) The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6:33. https://doi.org/10.1186/1471-230x-6-33
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Tirosh A, Shai I, Tekes-Manova D et al (2005) Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353:1454–1462. https://doi.org/10.1056/NEJMoa050080
Wang K, Yuan Y, Cho JH et al (2012) Comparing the microRNA spectrum between serum and plasma. PLoS One 7:e41561. https://doi.org/10.1371/journal.pone.0041561
Gong Q, Gregg EW, Wang J et al (2011) Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: China Da Qing Diabetes Prevention Outcome Study. Diabetologia 54:300–307. https://doi.org/10.1007/s00125-010-1948-9
Lindstrom J, Peltonen M, Eriksson JG et al (2013) Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study. Diabetologia 56:284–293. https://doi.org/10.1007/s00125-012-2752-5
He Y, Ding Y, Liang B et al (2017) A systematic study of dysregulated microRNA in type 2 diabetes. Int J Mol Sci 18:456. https://doi.org/10.3390/ijms18030456
Liang YZ, Li JJH, Xiao HB et al (2018) Identification of stress-related microRNA biomarkers in type 2 diabetes: a systematic review and meta-analysis. J Diabetes. https://doi.org/10.1111/1753-0407.12643
Lund AH (2010) miR-10 in development and cancer. Cell Death Differ 17:209–214. https://doi.org/10.1038/cdd.2009.58
Zhao X, Chen Z, Zhou Z et al (2019) High-throughput sequencing of small RNAs and analysis of differentially expressed microRNAs associated with high-fat diet-induced hepatic insulin resistance in mice. Genes Nutr 14:6. https://doi.org/10.1186/s12263-019-0630-1
Herrera BM, Lockstone HE, Taylor JM et al (2010) Global miRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53:1099–1109. https://doi.org/10.1007/s00125-010-1667-2
Wen D, Qiao P, Wang L (2015) Circulating miR-223 as a potential biomarker for obesity. Obes Res Clin Pract 9:398–404. https://doi.org/10.1016/j.orcp.2015.01.006
Ye D, Zhang T, Lou G, Liu Y (2018) Role of miR-223 in the pathophysiology of liver diseases. Exp Mol Med 50:128. https://doi.org/10.1038/s12276-018-0153-7
Aziz F (2016) The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol 303:1–6. https://doi.org/10.1016/j.cellimm.2016.04.003
Hay N (2011) Akt isoforms and glucose homeostasis. Trends Endocrinol Metab 22:66–73. https://doi.org/10.1016/j.tem.2010.09.003
Fabre A, Marchal S, Barlogis V et al (2019) Clinical aspects of STAT3 gain-of-function germline mutations. J Allergy Clin Immunol Pract 7:1958–1969. https://doi.org/10.1016/j.jaip.2019.02.018
Kung C-P, Murphy ME (2016) The role of the p53 tumor suppressor in metabolism and diabetes. J Endocrinol 231:R61–R75. https://doi.org/10.1530/JOE-16-0324
Moldovan L, Batte KE, Trgovcich J et al (2014) Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med 18:371–390. https://doi.org/10.1111/jcmm.12236
Venturella M, Carpi FM, Zocco D (2019) Standardization of blood collection and processing for the diagnostic use of extracellular vesicles. Curr Pathobiol Rep. https://doi.org/10.1007/s40139-019-00189-3
Blondal T, Jensby Nielsen S, Baker A et al (2013) Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 59:164–169. https://doi.org/10.1016/j.ymeth.2012.09.015
Acknowledgements
We are indebted to the IDIBAPS Biobank, integrated in the Spanish National Biobank Network, for the sample and data procurement. We are grateful to Yaiza Esteban and Lucía-Isabel Alvarado for technical support. We acknowledge the PREDAPS Study investigators: Gabriel Cuatrecasas (EAP Sarria SL), Eduard Tarragó (EAP Bellvitge), M. Isabel Bobé (EAP La Mina), Flora Lopez (EAP Martorell), Martí Birules (EAP Poble Nou), Rosamar DeMiguel (EAP Pubilla Casas), Laura Romera (EAP Raval Nord), Ana Martinez-Sanchez (EAP El Carmel), Belén Benito, Beatriz Bilbeny and Neus Piulachs (EAP Raval Sud).
Funding
This work was supported by grants EFSD/Lilly and an unrestricted grant from Novartis awarded to AN. It also received support from CIBERDEM.
Author information
Authors and Affiliations
Contributions
MP and AN designed the study; XM, XC, SC, LB, CGG, ER, MMC and JFN performed data collection and clinical analysis; CC and MP performed exosome isolation and RNA analysis; SC, MP and CC performed statistical analyses; MP wrote the first draft of the manuscript; all authors provided critical revisions of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were disclosed.
Ethical approval
The study was approved by the Ethical Committees of Parc de Salut Mar Clinical of Barcelona (Register 2011/4274/I) and the Hospital Clinic, University of Barcelona (Register 2011/6945).
Informed consent
Written informed consent was obtained from all the participants included in this study, in accordance with the principles of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parrizas, M., Mundet, X., Castaño, C. et al. miR-10b and miR-223-3p in serum microvesicles signal progression from prediabetes to type 2 diabetes. J Endocrinol Invest 43, 451–459 (2020). https://doi.org/10.1007/s40618-019-01129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01129-z