Abstract
Purpose
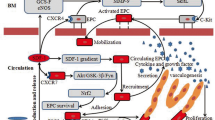
In peripheral artery disease, blockage of the blood supply to the limbs leads to blood flow attenuation and tissue ischemia. We investigated whether growth hormone-releasing hormone (GHRH) could enhance the biological functions and therapeutic effects of endothelial progenitor cells (EPCs) derived from adult human peripheral blood (PB).
Methods
EPCs were isolated from human PB (PB-EPCs) and cord blood and expanded in vitro. PB-EPCs incubated with or without GHRH were evaluated for proliferation, migration, and angiogenesis capacity and apoptosis rates under oxidative stress conditions. Activation of STAT3 and Akt pathways was evaluated using Western blot. A hind-limb ischemia (HLI) mouse model was used to study the efficacy of GHRH in improving EPC therapy in vivo.
Results
GHRH enhanced the proliferation, migration, and angiogenesis capacity of PB-EPCs and reduced apoptosis under H2O2 stimulation. These beneficial effects were GHRH receptor-dependent and were paralleled by increased phosphorylation of STAT3 and Akt. Transplantation of GHRH-preconditioned EPCs into HLI model mice enhanced blood flow recovery by increasing vascular formation density and enhanced tissue regeneration at the lesion site.
Conclusion
Our studies demonstrate a novel role for GHRH in dramatically improving therapeutic angiogenesis in HLI by enhancing the biological functions of EPCs. These findings support additional studies to explore the full potential of GHRH in augmenting cell therapy for the management of ischemia.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
28 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40618-022-01759-w
References
Abdulhannan P, Russell DA, Homer-Vanniasinkam S (2012) Peripheral arterial disease: a literature review. Br Med Bull 104:21–39. https://doi.org/10.1093/bmb/lds027
Selvin E, Erlinger TP (2004) Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 110(6):738–743. https://doi.org/10.1161/01.cir.0000137913.26087.f0
Lawall H, Bramlage P, Amann B (2010) Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemostasis 103(4):696–709. https://doi.org/10.1160/th09-10-0688
Williamson K, Stringer SE, Alexander MY (2012) Endothelial progenitor cells enter the aging arena. Front physiol 3:30. https://doi.org/10.3389/fphys.2012.00030
Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C (2005) Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45(9):1441–1448. https://doi.org/10.1016/j.jacc.2004.12.074
Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA (2003) Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 108(4):457–463. https://doi.org/10.1161/01.cir.0000082924.75945.48
Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO (1995) Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res 50:35–73
Muller EE, Locatelli V, Cocchi D (1999) Neuroendocrine control of growth hormone secretion. Physiol Rev 79(2):511–607. https://doi.org/10.1152/physrev.1999.79.2.511
Izdebski J, Pinski J, Horvath JE, Halmos G, Groot K, Schally AV (1995) Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA 92(11):4872–4876
Granata R, Trovato L, Gallo MP, Destefanis S, Settanni F, Scarlatti F, Brero A, Ramella R, Volante M, Isgaard J, Levi R, Papotti M, Alloatti G, Ghigo E (2009) Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res 83(2):303–312. https://doi.org/10.1093/cvr/cvp090
Schally AV, Perez R, Block NL, Rick FG (2015) Potentiating effects of GHRH analogs on the response to chemotherapy. Cell Cycle 14(5):699–704. https://doi.org/10.1080/15384101.2015.1010893
Penna C, Settanni F, Tullio F, Trovato L, Pagliaro P, Alloatti G, Ghigo E, Granata R (2013) GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology 154(4):1624–1635. https://doi.org/10.1210/en.2012-2064
Ma Q, Xia X, Tao Q, Lu K, Shen J, Xu Q, Hu X, Tang Y, Block NL, Webster KA, Schally AV, Wang J, Yu H (2016) Profound actions of an agonist of growth hormone-releasing hormone on angiogenic therapy by mesenchymal stem cells. Arterioscler Thromb Vasc Biol 36(4):663–672. https://doi.org/10.1161/ATVBAHA.116.307126
Shen J, Zhang N, Lin YN, Xiang P, Liu XB, Shan PF, Hu XY, Zhu W, Tang YL, Webster KA, Cai R, Schally AV, Wang J, Yu H (2018) Regulation of vascular calcification by growth hormone-releasing hormone and its agonists. Circ Res 122(10):1395–1408. https://doi.org/10.1161/circresaha.117.312418
Russell-Aulet M, Jaffe CA, Demott-Friberg R, Barkan AL (1999) In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab 84(10):3490–3497. https://doi.org/10.1210/jcem.84.10.6063
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Haider KH, Aziz S, Al-Reshidi MA (2017) Endothelial progenitor cells for cellular angiogenesis and repair: lessons learned from experimental animal models. Regen Med 12(8):969–982. https://doi.org/10.2217/rme-2017-0074
Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J (2007) Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res 100(3):434–443. https://doi.org/10.1161/01.RES.0000257912.78915.af
Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A (2005) Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 45(9):1449–1457. https://doi.org/10.1016/j.jacc.2004.11.067
Yuan Q, Hu CP, Gong ZC, Bai YP, Liu SY, Li YJ, Jiang JL (2015) Accelerated onset of senescence of endothelial progenitor cells in patients with type 2 diabetes mellitus: role of dimethylarginine dimethylaminohydrolase 2 and asymmetric dimethylarginine. Biochem Biophys Res Commun 458(4):869–876. https://doi.org/10.1016/j.bbrc.2015.02.050
Bakogiannis C, Tousoulis D, Androulakis E, Briasoulis A, Papageorgiou N, Vogiatzi G, Kampoli AM, Charakida M, Siasos G, Latsios G, Antoniades C, Stefanadis C (2012) Circulating endothelial progenitor cells as biomarkers for prediction of cardiovascular outcomes. Curr Med Chem 19(16):2597–2604
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353(10):999–1007. https://doi.org/10.1056/NEJMoa043814
Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J (2007) Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 115(14):1866–1875. https://doi.org/10.1161/circulationaha.106.659730
Haider H, Ashraf M (2010) Preconditioning and stem cell survival. J Cardiovasc Transl Res 3(2):89–102. https://doi.org/10.1007/s12265-009-9161-2
Lin-Su K, Wajnrajch MP (2002) Growth hormone releasing hormone (GHRH) and the GHRH receptor. Rev Endocr Metab Disord 3(4):313–323
Petersenn S, Schulte HM (2000) Structure and function of the growth-hormone-releasing hormone receptor. Vitam Horm 59:35–69
Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J (1995) Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology 136(9):4147–4150. https://doi.org/10.1210/endo.136.9.7649123
Suhr ST, Rahal JO, Mayo KE (1989) Mouse growth-hormone-releasing hormone: precursor structure and expression in brain and placenta. Mol Endocrinol 3(11):1693–1700. https://doi.org/10.1210/mend-3-11-1693
Siejka A, Schally AV, Barabutis N (2010) Activation of Janus kinase/signal transducer and activator of transcription 3 pathway by growth hormone-releasing hormone. Cell Mol Life Sci CMLS 67(6):959–964. https://doi.org/10.1007/s00018-009-0224-y
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399(6736):601–605. https://doi.org/10.1038/21224
Hausenloy DJ, Yellon DM (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12(3–4):217–234. https://doi.org/10.1007/s10741-007-9026-1
Hur J, Yoon CH, Lee CS, Kim TY, Oh IY, Park KW, Kim JH, Lee HS, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS (2007) Akt is a key modulator of endothelial progenitor cell trafficking in ischemic muscle. Stem Cells 25(7):1769–1778. https://doi.org/10.1634/stemcells.2006-0385
Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH (2005) STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J 19(10):1296–1298. https://doi.org/10.1096/fj.04-3099fje
Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR (2007) STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol 42(6):1009–1015. https://doi.org/10.1016/j.yjmcc.2007.04.010
Acknowledgements
This study was financially supported by Wenzhou Municipal Science and Technology Bureau Foundation (grant # Y20170008 and # Y20170052), Natural Science Foundation of Zhejiang Province (grant # LQ18H090007), and National Natural Science Foundation of China (grant # 81800314).
Funding
This study was funded by Wenzhou Municipal Science and Technology Bureau Foundation (grant # Y20170008), Natural Science Foundation of Zhejiang Province (grant # LQ18H090007), Department of Education of Zhejiang Province (Y201636426), and National Natural Science Foundation of China (grant # 81800314).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Ethical approval
The ethics committee of the First Affiliated Hospital of Wenzhou Medical University approved the study (2017-310).
Informed consent
All informed consents were obtained from all subjects according to the protocol approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (2017-310).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, N., Zhu, L. et al. Growth hormone-releasing hormone promotes therapeutic effects of peripheral blood endothelial progenitor cells in ischemic repair. J Endocrinol Invest 43, 315–328 (2020). https://doi.org/10.1007/s40618-019-01109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01109-3