Abstract
Purpose
Hashimoto’s thyroiditis (HT) is the most common form of autoimmune thyroid diseases. Current knowledge of HT genetics is limited, and not a single genome-wide association study (GWAS) focusing exclusively on HT has been performed to date. In order to decipher genetic determinants of HT, we performed the first GWAS followed by replication in a total of 1443 individuals from Croatia.
Methods
We performed association analysis in a discovery cohort comprising 405 cases and 433 controls. We followed up 13 independent signals (P < 10−5) in 303 cases and 302 controls from two replication cohorts and then meta-analyzed results across discovery and replication datasets.
Results
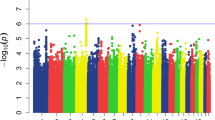
We identified three variants suggestively associated with HT: rs12944194 located 206 kb from SDK2 (P = 1.8 × 10−6), rs75201096 inside GNA14 (P = 2.41 × 10−5) and rs791903 inside IP6K3 (P = 3.16 × 10−5). Genetic risk score (GRS), calculated using risk alleles of these loci, accounted for 4.82% of the total HT variance, and individuals from the top GRS quartile had 2.76 times higher odds for HT than individuals from the lowest GRS quartile.
Conclusions
Although discovered loci are implicated with susceptibility to HT for the first time, genomic regions harboring these loci exhibit good biological candidacy due to involvement in the regulation of the thyroid function and autoimmunity. Additionally, we observe genetic overlap between HT and several related traits, such as hypothyroidism, Graves’ disease and TPOAb. Our study adds a new knowledge of underlying HT genetics and sets a firm basis for further research.

Similar content being viewed by others
References
Xiaoheng C, Yizhou M, Bei H et al (2017) General and specific genetic polymorphism of cytokines-related gene in AITD. Mediators Inflamm 2017:3916395. https://doi.org/10.1155/2017/3916395
McGrogan A, Seaman HE, Wright JW, de Vries CS (2008) The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol 69(5):687–696. https://doi.org/10.1111/j.1365-2265.2008.03338.x
Caturegli P, De Remigis A, Rose NR (2014) Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev 13(4–5):391–397. https://doi.org/10.1016/j.autrev.2014.01.007
Dayan CM, Daniels GH (1996) Chronic autoimmune thyroiditis. N Engl J Med 335(2):99–107. https://doi.org/10.1056/NEJM199607113350206
Zaletel K, Gaberscek S (2011) Hashimoto’s thyroiditis: from genes to the disease. Curr Genom 12(8):576–588. https://doi.org/10.2174/138920211798120763
Zaletel K (2007) Determinants of thyroid autoantibody production in Hashimoto’s thyroiditis. Exp Rev Clin Immunol 3(2):217–223. https://doi.org/10.1586/1744666X.3.2.217
McLachlan SM, Rapoport B (2004) Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid Off J Am Thyroid Assoc 14(7):510–520. https://doi.org/10.1089/1050725041517057
Brix TH, Hegedus L (2012) Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol 76(4):457–464. https://doi.org/10.1111/j.1365-2265.2011.04318.x
Jabrocka-Hybel A, Skalniak A, Piatkowski J et al (2018) How much of the predisposition to Hashimoto’s thyroiditis can be explained based on previously reported associations? J Endocrinol Invest. https://doi.org/10.1007/s40618-018-0910-4
Ajjan RA, Weetman AP (2015) The Pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 47(10):702–710. https://doi.org/10.1055/s-0035-1548832
Denny JC, Crawford DC, Ritchie MD et al (2011) Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet 89(4):529–542. https://doi.org/10.1016/j.ajhg.2011.09.008
Eriksson N, Tung JY, Kiefer AK et al (2012) Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One 7(4):e34442. https://doi.org/10.1371/journal.pone.0034442
Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA (2016) Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 48(7):709–717. https://doi.org/10.1038/ng.3570
Cooper JD, Simmonds MJ, Walker NM et al (2012) Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet 21(23):5202–5208. https://doi.org/10.1093/hmg/dds357
Oryoji D, Ueda S, Yamamoto K et al (2015) Identification of a Hashimoto thyroiditis susceptibility locus via a genome-wide comparison with Graves’ disease. J Clin Endocrinol Metab 100(2):E319–E324. https://doi.org/10.1210/jc.2014-3431
Brcic L, Baric A, Gracan S et al (2016) Association of established thyroid peroxidase autoantibody (TPOAb) genetic variants with Hashimoto’s thyroiditis. Autoimmunity 49(7):480–485. https://doi.org/10.1080/08916934.2016.1191475
Baric A, Brcic L, Gracan S et al (2017) Association of established hypothyroidism-associated genetic variants with Hashimoto’s thyroiditis. J Endocrinol Invest 40(10):1061–1067. https://doi.org/10.1007/s40618-017-0660-8
Brcic L, Gracan S, Baric A et al (2017) Association of established thyroid-stimulating hormone and free thyroxine genetic variants with Hashimoto’s thyroiditis. Immunol Invest 46(6):625–638. https://doi.org/10.1080/08820139.2017.1337785
Rudan I, Marusic A, Jankovic S et al (2009) “10001 Dalmatians:” Croatia launches its national biobank. Croat Med J 50(1):4–6
Delaneau O, Zagury JF, Marchini J (2013) Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 10(1):5–6. https://doi.org/10.1038/nmeth.2307
Howie BN, Donnelly P, Marchini J (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5(6):e1000529. https://doi.org/10.1371/journal.pgen.1000529
Zhou X, Stephens M (2012) Genome-wide efficient mixed-model analysis for association studies. Nat Genet 44(7):821–824. https://doi.org/10.1038/ng.2310
Stefanic M, Papic S, Suver M, Glavas-Obrovac L, Karner I (2008) Association of vitamin D receptor gene 3′-variants with Hashimoto’s thyroiditis in the Croatian population. Int J Immunogenet 35(2):125–131. https://doi.org/10.1111/j.1744-313X.2008.00748.x
Lessel D, Vaz B, Halder S et al (2014) Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet 46(11):1239–1244. https://doi.org/10.1038/ng.3103
Pruim RJ, Welch RP, Sanna S et al (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26(18):2336–2337. https://doi.org/10.1093/bioinformatics/btq419
Ji R, Feng Y, Zhan WW (2013) Updated analysis of studies on the cytotoxic T-lymphocyte-associated antigen-4 gene A49G polymorphism and Hashimoto’s thyroiditis risk. Genet Mol Res GMR 12(2):1421–1430. https://doi.org/10.4238/2013.April.26.4
Santos LR, Duraes C, Mendes A et al (2014) A polymorphism in the promoter region of the selenoprotein S gene (SEPS1) contributes to Hashimoto’s thyroiditis susceptibility. J Clin Endocrinol Metab 99(4):E719–E723. https://doi.org/10.1210/jc.2013-3539
Porcu E, Medici M, Pistis G et al (2013) A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 9(2):e1003266. https://doi.org/10.1371/journal.pgen.1003266
Tylee DS, Sun J, Hess JL et al. (2018) Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. BioRxiv. https://doi.org/10.1101/070730
Ahola-Olli AV, Wurtz P, Havulinna AS et al (2017) Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet 100(1):40–50. https://doi.org/10.1016/j.ajhg.2016.11.007
Lauc G, Huffman JE, Pucic M et al (2013) Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet 9(1):e1003225. https://doi.org/10.1371/journal.pgen.1003225
Batut P, Dobin A, Plessy C, Carninci P, Gingeras TR (2013) High-fidelity promoter profiling reveals widespread alternative promoter usage and transposon-driven developmental gene expression. Genome Res 23(1):169–180. https://doi.org/10.1101/gr.139618.112
An integrated encyclopedia of DNA elements in the human genome (2012). Nature 489(7414):57–74. https://doi.org/10.1038/nature11247
Kero J, Ahmed K, Wettschureck N et al (2007) Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Investig 117(9):2399–2407. https://doi.org/10.1172/JCI30380
Arnaud-Lopez L, Usala G, Ceresini G et al (2008) Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet 82(6):1270–1280. https://doi.org/10.1016/j.ajhg.2008.04.019
Okada Y, Wu D, Trynka G et al (2014) Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506(7488):376–381. https://doi.org/10.1038/nature12873
Boelaert K, Newby PR, Simmonds MJ et al (2010) Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 123(2):183 e181–189. https://doi.org/10.1016/j.amjmed.2009.06.030
Bliddal S, Borresen SW, Feldt-Rasmussen U (2017) Thyroid autoimmunity and function after treatment with biological antirheumatic agents in rheumatoid arthritis. Front Endocrinol 8:179. https://doi.org/10.3389/fendo.2017.00179
Nakabayashi K, Tajima A, Yamamoto K et al (2011) Identification of independent risk loci for Graves’ disease within the MHC in the Japanese population. J Hum Genet 56(11):772–778. https://doi.org/10.1038/jhg.2011.99
Simmonds MJ (2013) GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol 9(5):277–287. https://doi.org/10.1038/nrendo.2013.56
Funding
Formation of HT Biobank and genome-wide genotyping of patients with HT was supported by the Croatian Science Foundation (Grant no. 4950). The “10 001 Dalmatians” project was supported by the MRC Human Genetics Unit, The Croatian Ministry of Science, Education and Sports (Grant 216-1080315-0302), the European Union framework program 6 EUROSPAN project (Contract no. LSHG-CT-2006-018947), EU FP7 BBMRI-LPC (Biobanking and biomolecular resources research infrastructure—Large prospective cohort, Contract 313010) and the Croatian Science Foundation (Grant nos. 8875 and 1498).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Study was approved by two separate Ethics Committees: the University of Split, School of Medicine (Classification no. 003-08/14-03/0001; Registry no. 2181-198-03-04-14-0028) and University Hospital Split (Classification no. 530-02/13-01/11; Registry no. 2181-147-01/06/J.B.-14-2).
Informed consent
Written informed consent was obtained from all study participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brčić, L., Barić, A., Gračan, S. et al. Genome-wide association analysis suggests novel loci for Hashimoto’s thyroiditis. J Endocrinol Invest 42, 567–576 (2019). https://doi.org/10.1007/s40618-018-0955-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0955-4